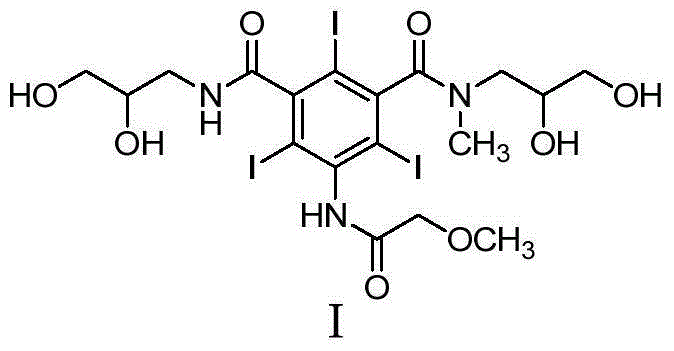
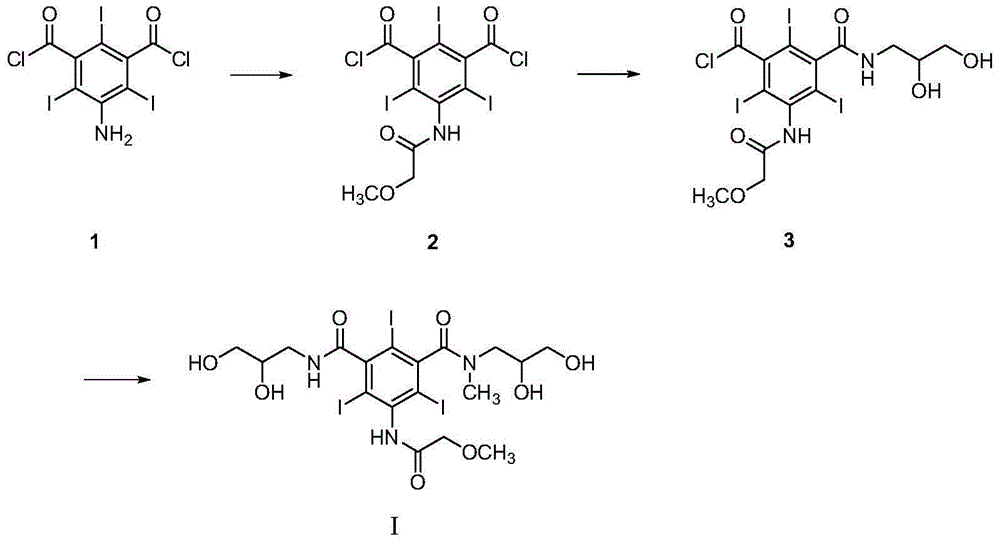
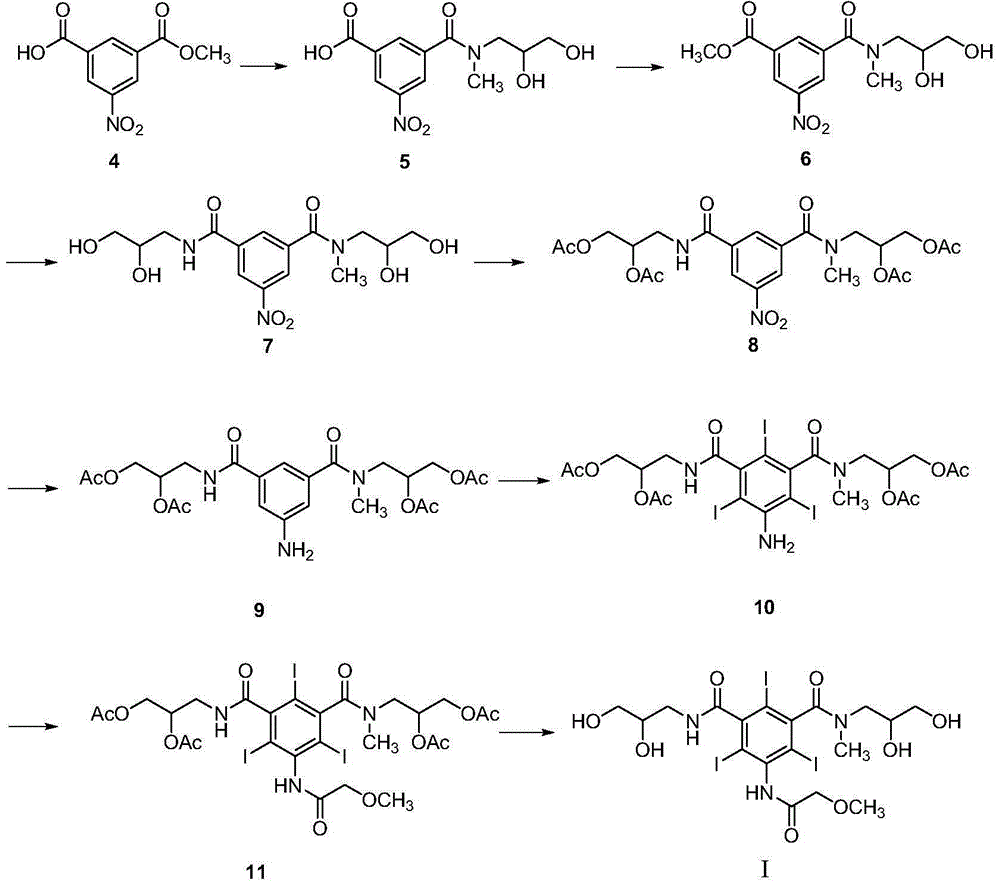
Preparation method and intermediates of iopromide
An iopromide and compound technology, which is applied in the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem that acetyl groups are easily removed, acetyl protecting groups are easily removed, and are unfavorable for industrial production. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Example 1: Preparation of 3-((2,3-dihydroxypropyl)carbamoyl)-5-nitrobenzoic acid (Formula III-1)
[0093]
[0094] Add 3-methoxycarbonyl-5-nitrobenzoic acid (Formula II, 45g, 0.2mol) into the reaction flask, add acetonitrile (180mL) to dissolve it, then add 3-amino-1,2-propanediol (54.6g, 0.6mol), the temperature of the reaction solution was raised to 75-80°C, and the reaction was carried out for 7 hours. After the reaction, the lower layer of the reaction solution was added to 500ml of 1N hydrochloric acid, crystallized overnight at 0-10°C, filtered, and the filter cake was washed once with an appropriate amount of water and ethyl acetate to obtain 3-((2,3-dihydroxy Propyl)carbamoyl)-5-nitrobenzoic acid (Formula III-1, 46.6g), yield 82%, purity ≥ 99%.
Embodiment 2
[0095] Example 2: Preparation of 3-nitro-5-(((2-oxo-1,3-dioxolan-4-yl)methyl)carbamoyl)benzoic acid (Formula IV-1)
[0096]
[0097] Dissolve 3-((2,3-dihydroxypropyl)carbamoyl)-5-nitrobenzoic acid (Formula III-1, 28.4g, 0.1mol) in DMF (60mL), then add triethylamine (10.1g, 0.1mol), the temperature of the reaction solution was cooled to 0°C, and then CDI (24.3g, 0.15mol) was added in batches, the reaction solution was warmed to room temperature, and reacted for 1 hour. After the reaction, the reaction solution was added to 500ml of 1N hydrochloric acid for precipitation, filtered, and the filter cake was washed once with water to obtain 3-nitro-5-(((2-oxo-1,3-dioxolan-4-yl) Methyl)carbamoyl)benzoic acid (Formula IV-1, 19.2g), the yield is 62%, and the purity is ≥98%.
[0098] MS m / z[ESI]:311.0[M+1] + .
Embodiment 3
[0099] Embodiment three: N 1 -(2,3-Dihydroxypropyl)-N 1 -Methyl-5-nitro-N 3 Preparation of -((2-oxo-1,3-dioxolan-4-yl)methyl)isophthalamide (formula Ⅴ-1)
[0100]
[0101] Add 3-nitro-5-(((2-oxo-1,3-dioxolan-4-yl)methyl)carbamoyl)benzoic acid (formula IV-1, 31g, 0.1mol ), added dichloromethane (100mL) to dissolve it, the reaction solution was cooled to 0°C, and oxalyl chloride (19.05g, 0.15mol) was added dropwise. The solution was concentrated, and the concentrate was redissolved with dichloromethane (100 mL). Dissolve 3-methylamino-1,2-propanediol (26.25g, 0.25mol) in ethanol (50mL), and add the above reconstituted acid chloride solution dropwise at -10°C. The whole dropping process is maintained for 2 hours, and the dropping is completed Stirring was then continued for 0.5 hours. After the reaction, the reaction solution was washed with water, the organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated to obtain N 1 -(2,3-D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com