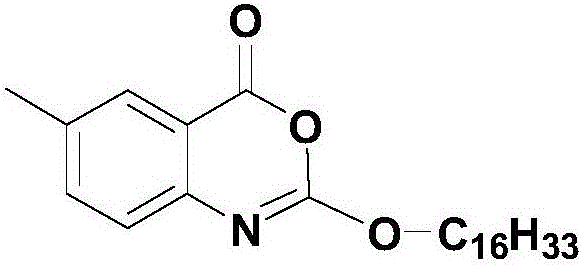
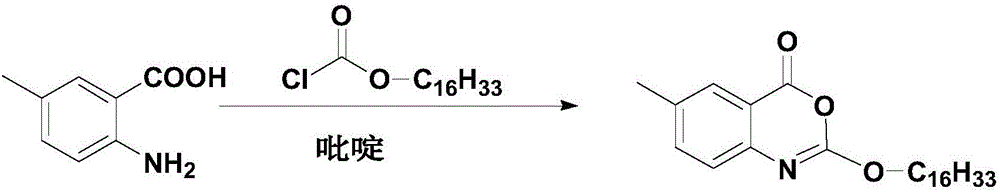
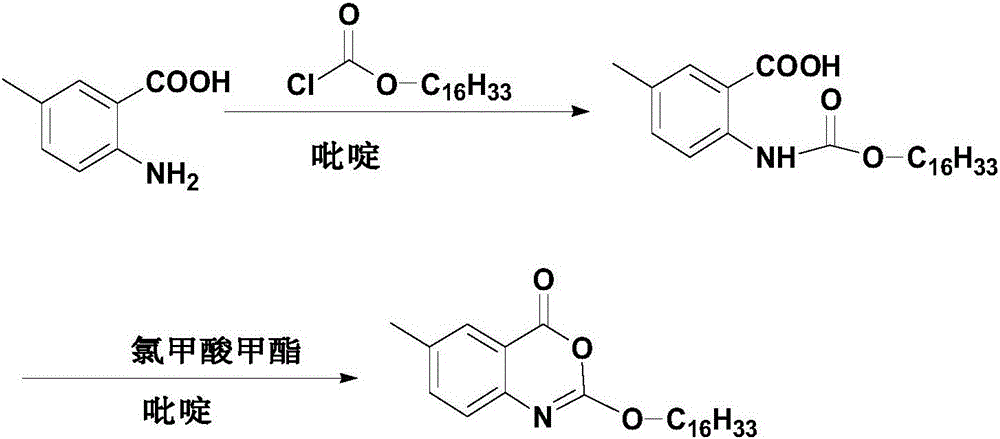
Cetilistat preparation method
A technology of new lisstat and synthesis method, which is applied in the field of preparation of new lisstat, a drug for the treatment of obesity and its complications, can solve the problems of high equipment requirements, complicated operation, high risk factor, etc., and achieve economical and safe Improvement, post-processing is easy to operate, and the effect of low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Weigh 12g (79.39mmol, 1.0eq) of 2-amino-5-methylbenzoic acid, 10.03ml (91.31mmol, 1.15eq) of N-methylmorpholine and join in a 500ml three-necked flask, add 100ml absolute ethanol, Stir to dissolve, and cool down in an ice bath. When the temperature of the reaction system is 5°C, start to slowly add 29.05g (95.27mmol, 1.2eq) of hexadecyl chloroformate dropwise, and control the temperature not to exceed 10°C. The dropwise addition is completed in about 30 minutes , a large number of light yellow solids are formed in the system, slowly raise the temperature to 30°C, keep the temperature for 30 minutes, then cool down to 0-5°C, start to slowly add 18.12ml (223.86mmol, 3.0eq) of sulfuryl chloride dropwise, and the dropwise addition is completed in about 20 minutes , during the dropping process, the temperature is controlled not to exceed 10°C. After the dropping is completed, continue to stir and react for 30 minutes, then slowly raise the temperature to 25°, and keep the tem...
Embodiment 2
[0037] Weigh 12g (79.39mmol, 1.0eq) of 2-amino-5-methylbenzoic acid, 10.03ml (91.31mmol, 1.15eq) of N-methylmorpholine and join in a 500ml three-necked flask, add 100ml of anhydrous methanol, Stir to dissolve, and cool down in an ice bath. When the temperature of the reaction system is 5°C, start to slowly add 29.05g (95.27mmol, 1.2eq) of hexadecyl chloroformate dropwise, and control the temperature not to exceed 10°C. The dropwise addition is completed in about 30 minutes , a large number of light yellow solids are formed in the system, slowly raise the temperature to 30°C, keep the temperature for 30 minutes, then cool down to 0-5°C, start to slowly add 18.12ml (223.86mmol, 3.0eq) of sulfuryl chloride dropwise, and the dropwise addition is completed in about 20 minutes , during the dropping process, the temperature is controlled not to exceed 10°C. After the dropping is completed, continue to stir and react for 30 minutes, then slowly raise the temperature to 25°, and keep th...
Embodiment 3
[0039] Weigh 12g (79.39mmol, 1.0eq) of 2-amino-5-methylbenzoic acid, 10.03ml (91.31mmol, 1.15eq) of N-methylmorpholine and join in a 500ml three-necked flask, add 100ml isopropanol, Stir to dissolve, and cool down in an ice bath. When the temperature of the reaction system is 5°C, start to slowly add 29.05g (95.27mmol, 1.2eq) of hexadecyl chloroformate dropwise, and control the temperature not to exceed 10°C. The dropwise addition is completed in about 30 minutes , a large number of light yellow solids are formed in the system, slowly raise the temperature to 30°C, keep the temperature for 30 minutes, then cool down to 0-5°C, start to slowly add 18.12ml (223.86mmol, 3.0eq) of sulfuryl chloride dropwise, and the dropwise addition is completed in about 20 minutes , during the dropping process, the temperature is controlled not to exceed 10°C. After the dropping is completed, continue to stir and react for 30 minutes, then slowly raise the temperature to 25°, and keep the temperat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com