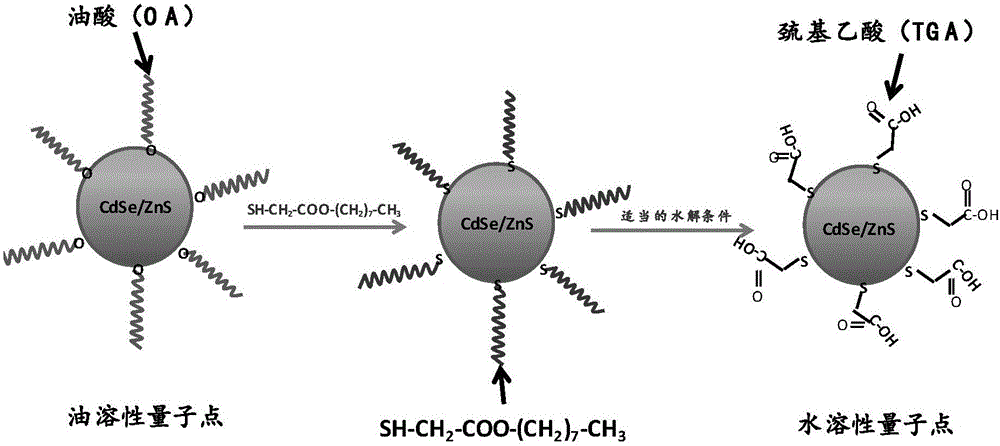
Quantum dot ligand exchange method
A ligand exchange, quantum dot technology, applied in chemical instruments and methods, nanotechnology for materials and surface science, nanotechnology, etc. effect and other problems, so as to solve the insufficient exchange of surface ligands, improve the coordination effect, and avoid the effect of agglomeration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
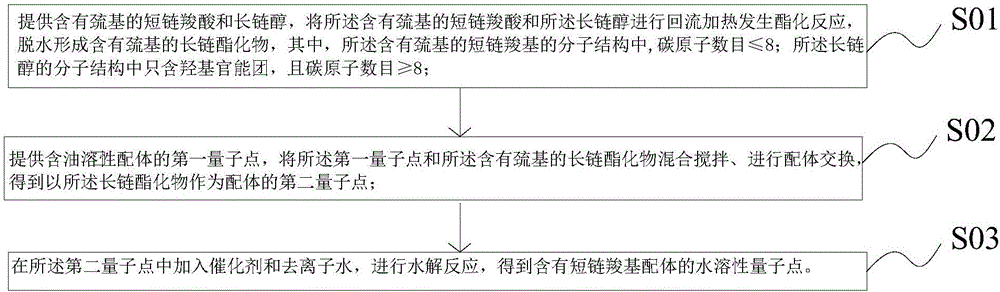
[0037] A method of quantum dot ligand exchange includes the following steps:
[0038] S11. Add 18.2g of mercaptoacetic acid, 28g of n-octanol, 1.4g of catalyst (80% sulfuric acid by mass), and 18ml of cyclohexane into a 250ml three-necked flask equipped with a thermometer, a water trap and a reflux condenser, and stir to reflux. After being heated to 96°C, thermostatic treatment was carried out, and the water generated during the reaction was continuously separated by a moisture container during the reaction. When the reaction did not produce any more water, the reflux was continued for 15 minutes, then the stirring was stopped, and the reaction was cooled to room temperature. After the reaction system is allowed to stand, the sulfuric acid in the bottom layer is separated; the organic layer is first neutralized with a 4% sodium carbonate solution, and then washed with hot water at 80°C. Pressure distillation, steam cyclohexane and n-octanol can be reused, and collect the n-octyl...
Embodiment 2
[0044] S21. Add 18.2g of thioglycolic acid, 58g of tridecanol, 1.4g of catalyst (80% sulfuric acid by mass), and 18ml of cyclohexane into a 250ml three-necked flask equipped with a thermometer, moisture device and reflux condenser, and stir to reflux. After being heated to 96°C, thermostatic treatment was carried out, and the water generated during the reaction was continuously separated by a moisture container during the reaction. When the reaction did not produce any more water, the reflux was continued for 15 minutes, then the stirring was stopped, and the reaction was cooled to room temperature. After the reaction system is allowed to stand, the sulfuric acid in the bottom layer is separated; the organic layer is first neutralized with a 4% sodium carbonate solution, and then washed with hot water at 80°C. Pressure distillation, steam cyclohexane and tridecanol can be reused, and collect n-octyl thioglycolate fraction under 132~136℃ / 2Kpa. The reaction equation for this step...
Embodiment 3
[0049] S31. Add 20.5g of mercaptooctanoic acid, 38g of n-octanol, 1.4g of catalyst (80% sulfuric acid by mass), and 18ml of cyclohexane into a 250ml three-necked flask equipped with a thermometer, a water trap and a reflux condenser, and stir to reflux. After being heated to 96°C, thermostatic treatment was carried out, and the water generated during the reaction was continuously separated by a moisture container during the reaction. When the reaction did not produce any more water, the reflux was continued for 15 minutes, then the stirring was stopped, and the reaction was cooled to room temperature. After the reaction system is allowed to stand, the sulfuric acid in the bottom layer is separated; the organic layer is first neutralized with a 4% sodium carbonate solution, and then washed with hot water at 80°C. Pressure distillation, steam cyclohexane and n-octanol can be reused, and collect the n-octyl mercaptooctanoate fraction at 132~136℃ / 2Kpa. The reaction equation for thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com