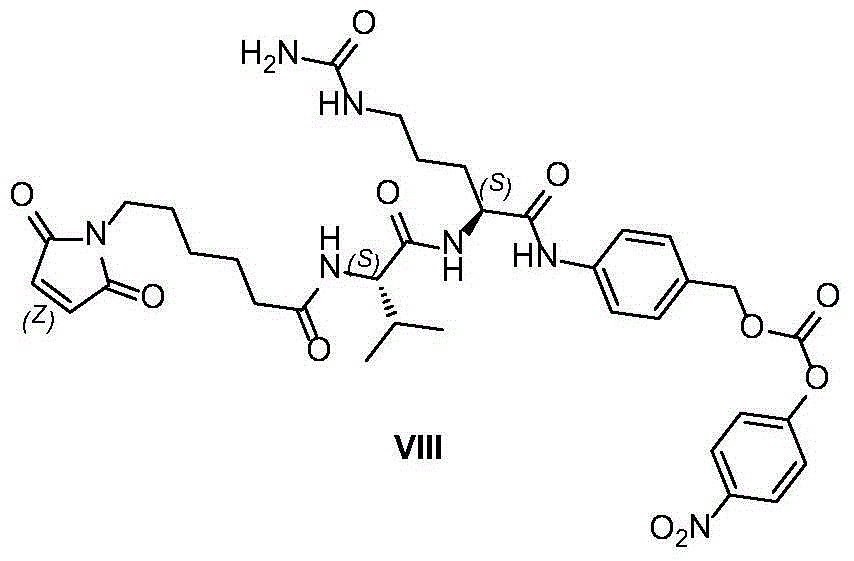
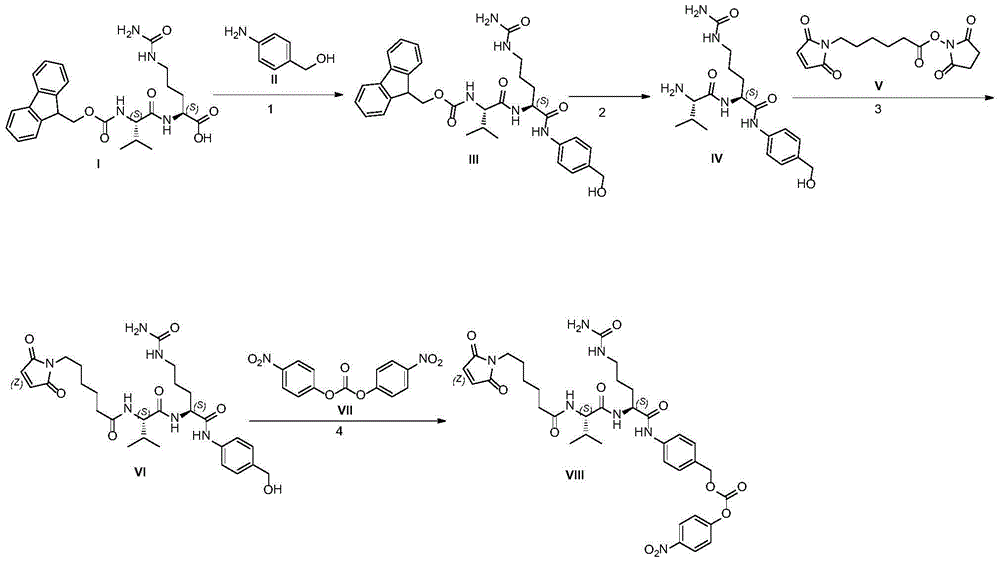
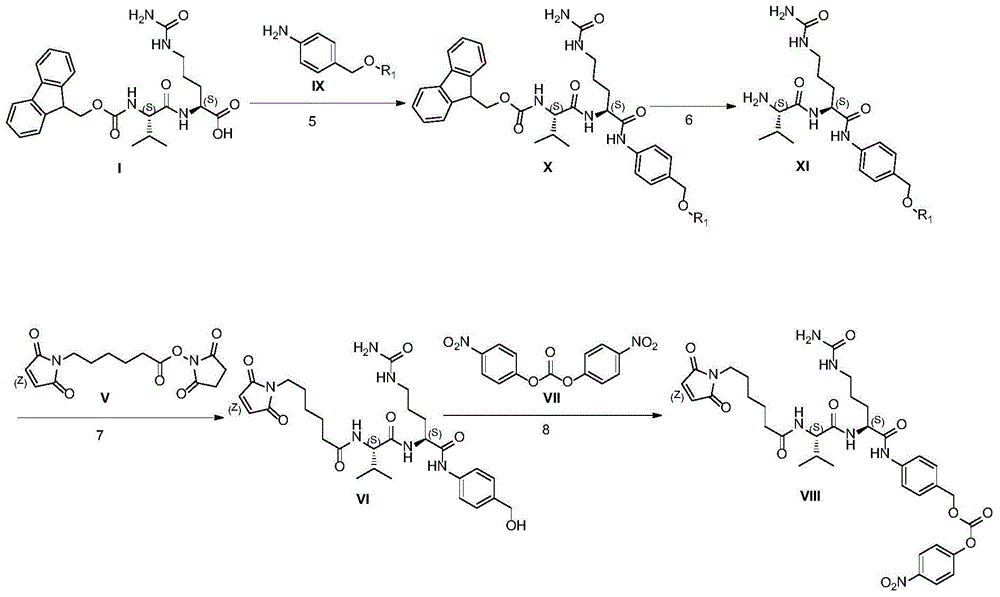
Industrialized production method for antibody coupling medicine connexon
A production method and linker technology, applied in the field of preparation of antibody-conjugated drug Adcetris linker, to achieve the effects of mild reaction conditions, simple and easy post-processing operation, and simple and convenient operation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: (9H-fluoren-9-yl)methyl ((S)-1-(((S)-1-((4-(((tert-butyldimethylsilyl)oxy) Preparation of methyl)phenyl)amino)-1-oxo-5-ureido-pent-2-yl)amino)-3-methyl-1-oxobutan-2-yl)carbamic acid
[0059]
[0060] Under the protection of nitrogen, compound I (1.08kg, 2.18mol, 1.0eq) and compound IX-1 (517.6g, 2.18mol, 1.0eq) were added to ethyl acetate (20.0L), stirred at room temperature for half an hour to the raw material All dissolved, then ethyl chloroformate (236.6g, 2.18mol, 1.0eq) and ethyl acetate (1.6L) solution of triethylamine (220.6g, 2.18mol, 1.0eq) were added to the reaction system, dropwise The process temperature is controlled at -5~-15°C. After the dropwise addition, slowly rise to room temperature and continue the reaction until the liquid phase tracking shows that the raw material I in the reaction system disappears, and the reaction is stopped. After the reaction was terminated, the solution was removed under normal pressure, the remaining solid w...
Embodiment 2
[0062] Example 2: (S)-2-((S)-2-amino-3-methylbutanamido)-N-(4-(((tert-butyldimethylsilyl)oxy) Preparation of Methyl)phenyl)-5-ureidopentanamide
[0063]
[0064] Add compound X-1 (3.44kg, 4.8mol, 1.0eq) to N,N-dimethylformamide (17.2L) at room temperature, stir for half an hour until all the raw materials are dissolved, then add diethylamine (700.8g , 9.6mol, 2.0eq), until the liquid phase tracking shows that the raw material X-1 in the reaction system disappears, stop the reaction. After the reaction is stopped, desolventize under normal pressure (spin off 3 / 4 of the total volume of the solution), then add the residue to ethyl acetate (5L), stir until clear, then add petroleum ether dropwise to the system until a white turbid solution appears , then add ethyl acetate back dropwise until just dissolved, leave it open at room temperature for 16-48 hours, solids precipitate out, filter, collect the filter cake and wash with petroleum ether (3L × 2), dry in vacuo to obtain a ...
Embodiment 3
[0066] Example 3: 6-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-N-((S)-1-(((S)-1-(( Preparation of 4-(hydroxymethyl)phenyl)amino)-1-oxo-5-ureidopent-2-yl)amino)-3-methyl-1-oxobutan-2-yl)hexanamide
[0067]
[0068] Compound XI-1 (269g, 0.55mol, 1.0eq) and compound V (168.1g, 0.55mol, 1.0eq) were added to N,N-dimethylformamide (1L) at room temperature, and stirred at room temperature for half an hour to All the raw materials were dissolved, and then the temperature was raised to 45-50°C and reacted for 4-6 hours to obtain a reaction liquid of the substitute, and the reaction liquid was cooled to -5-0°C and tetramethylammonium fluoride (50.7g, 0.55mol, 1.0eq ) in N,N-dimethylformamide (350 mL) solution, the dropwise addition was completed, and the temperature was raised to room temperature to react until the liquid phase tracking showed that the raw materials in the reaction system disappeared, and the reaction was stopped. After the reaction stopped, water was added to the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com