Method for producing cyanogen chloride by using tubular reactor
A tubular reactor, cyanogen chloride technology, applied in chemical instruments and methods, cyanogen halide, cyanide and other directions, can solve the problems of difficult control of gas-liquid mixing effect, strict spray control requirements, short process routes, etc. The effect of investment in fixed assets, simplified reaction equipment, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
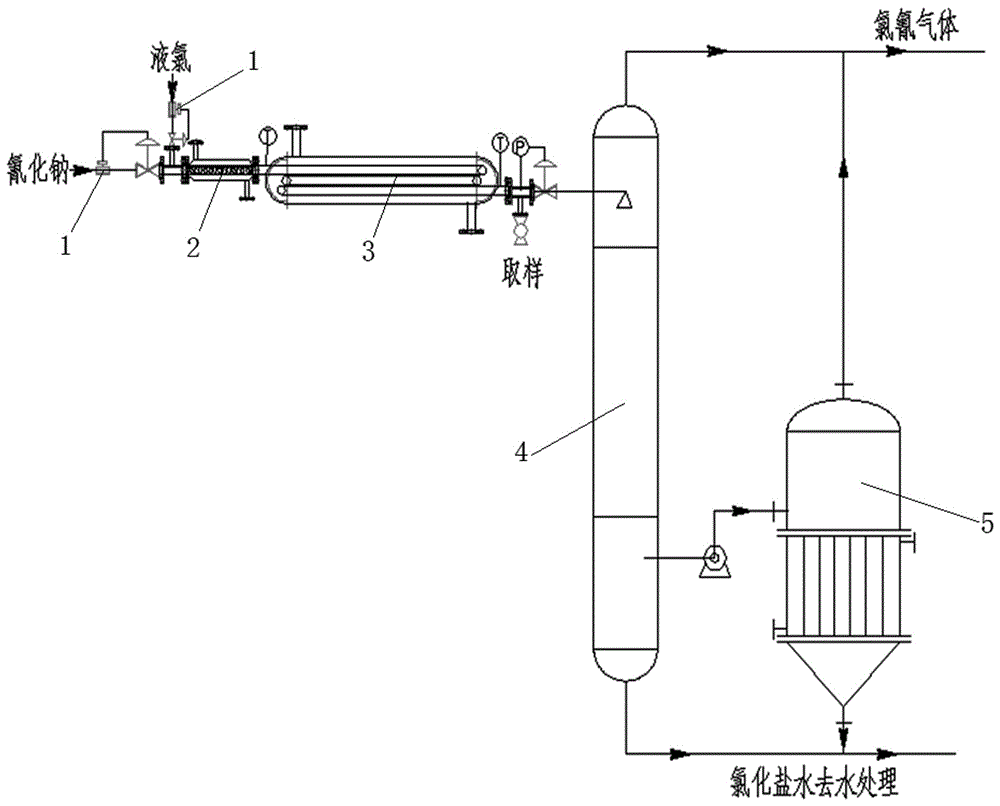
[0020] (1) After the flow is precisely adjusted by the intelligent flowmeter, the flow rate of liquid chlorine is controlled at 0.25t / h, and the flow rate of 30% sodium cyanide aqueous solution is controlled at 0.5t / h, respectively pass through their respective liquid feed pumps and enter the mixer for mixing. Control the outlet temperature of the mixer at 30°C, and control the molar ratio of sodium cyanide and liquid chlorine to 1:1.15;
[0021] (2) Introduce the mixed feed liquid into the tubular reactor, control the pressure of the tubular reactor to 0.8MPa, control the reaction temperature at 40°C with circulating water, and the reaction residence time is 30s, and take samples for detection;
[0022] (3) After the reaction is completed, the feed liquid enters the desorption tower from the end of the tubular reactor. The temperature of the desorption tower is controlled at 50°C. After desorption and drying, pure cyanogen chloride gas is obtained, and the conversion rate reac...
Embodiment 2
[0024] (1) After the flow is precisely adjusted by the intelligent flowmeter, the flow rate of liquid chlorine is controlled at 0.5t / h, and the flow rate of 30% sodium cyanide aqueous solution is controlled at 1.1t / h, which respectively pass through the respective liquid feed pumps and enter the mixer for mixing. Control the outlet temperature of the mixer at 40°C, and control the molar ratio of sodium cyanide and liquid chlorine to 1:1.045;
[0025] (2) Introduce the mixed feed liquid into the tubular reactor, control the pressure of the tubular reactor to 1.2MPa, control the reaction temperature at 45°C with circulating water, and the reaction residence time is 50s, and take samples for detection;
[0026] (3) After the reaction is completed, the feed liquid enters the desorption tower from the end of the tubular reactor. The temperature of the desorption tower is controlled at 60°C. After desorption and drying, pure cyanogen chloride gas is obtained, and the conversion rate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com