Application of fluorine-containing catechol structure compound serving as mycobacterium tuberculosis inhibitor
A technology of mycobacterium tuberculosis and catechol, which is applied in the fields of biomedicine and chemistry, can solve the problems of long treatment cycle and achieve the effect of inhibiting proliferation and increasing expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
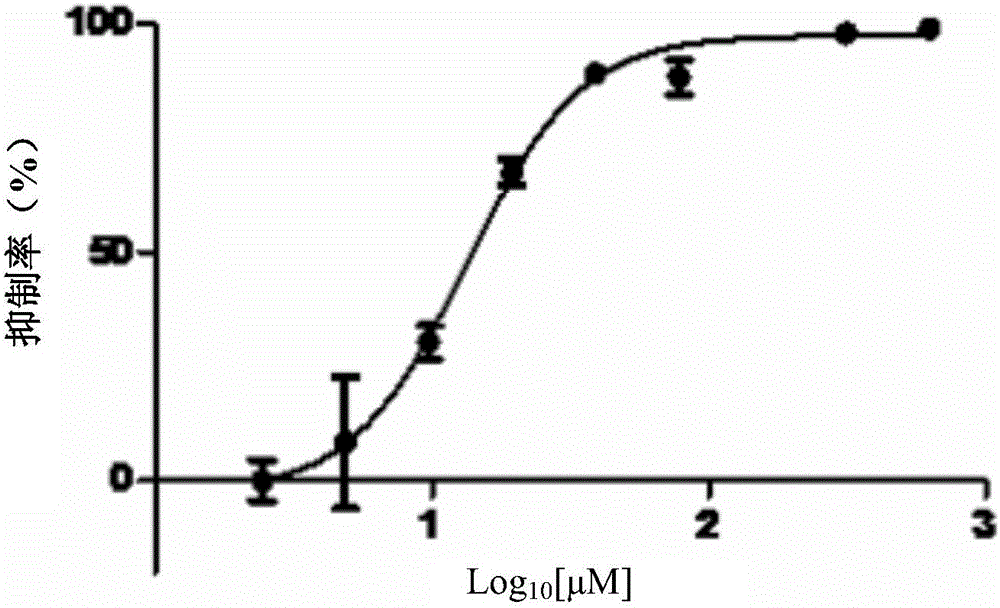
[0035] Embodiment 1, the direct inhibitory action of test drug to Mycobacterium tuberculosis
[0036] Test drug: 3-fluorocatechol, product of Sigma-Aldrich, product number 344656.
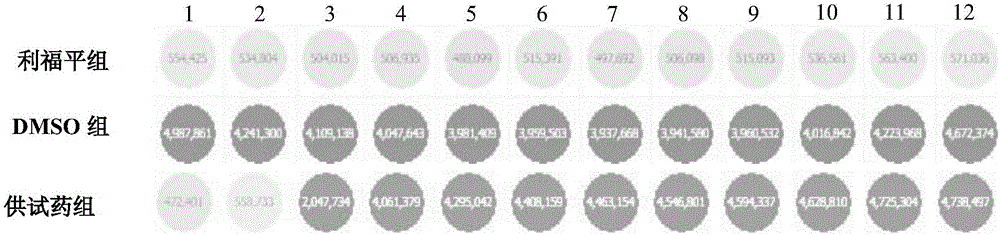
[0037] Adjust the concentration of Mycobacterium bovis BCG 1173P2 to OD 600 The reading value is 0.05 (corresponding to 1.5×10 7 CFU / mL), seeded in 96-well plate, 99 μL per well. Add 1.0 μL of the 2-fold concentration gradient dilution solution of the test drug to each well, and incubate at 37°C for 72 hours. 4.879 μM, 9.758 μM, 19.516 μM, 39.032 μM, 78.064 μM, 156.128 μM, 312.256 μM, 624.512 μM; at the same time set positive control rifampicin group (that is, there is BCG 1173P2 strain in the incubation system, but no test drug is added, and was added rifampicin), negative control dimethyl sulfoxide group (DMSO group, only 1 μL of DMSO was added). put each group in The fluorescence value of GFP was detected under the Multilabel Reader fluorescence microplate reader, the excitation wavelength...
Embodiment 2
[0039] Example 2, the inhibitory effect of the test drug on Mycobacterium tuberculosis in macrophages
[0040] Test drug: 3-fluorocatechol, product of Sigma-Aldrich, product number 344656.
[0041] THP-1 cells were divided into 5×10 5Cells / mL were inoculated in 96-well plates, 100 μL per well, differentiated with phorbol ester (PMA) at a final concentration of 100 ng / mL for 24 hours, and then the cells adhered to the wall to obtain macrophages derived from THP-1 cells. Macrophages derived from THP-1 cells were infected with Mycobacterium bovis BCG 1173P2 at an infectivity of MOI=10 for 4 hours. Wash away extracellular BCG 1173P2 with PBS, add 199 μL of 1640 medium containing 10% (volume fraction) fetal bovine serum, and then add 1 μL of each test drug at different concentrations, so that the final concentrations in the system are 2.440 μM and 4.879 μM respectively , 9.758 μM, 19.516 μM, 39.032 μM, 78.064 μM, 156.128 μM, 312.256 μM, 624.512 μM, continue to incubate for 48 hou...
Embodiment 3
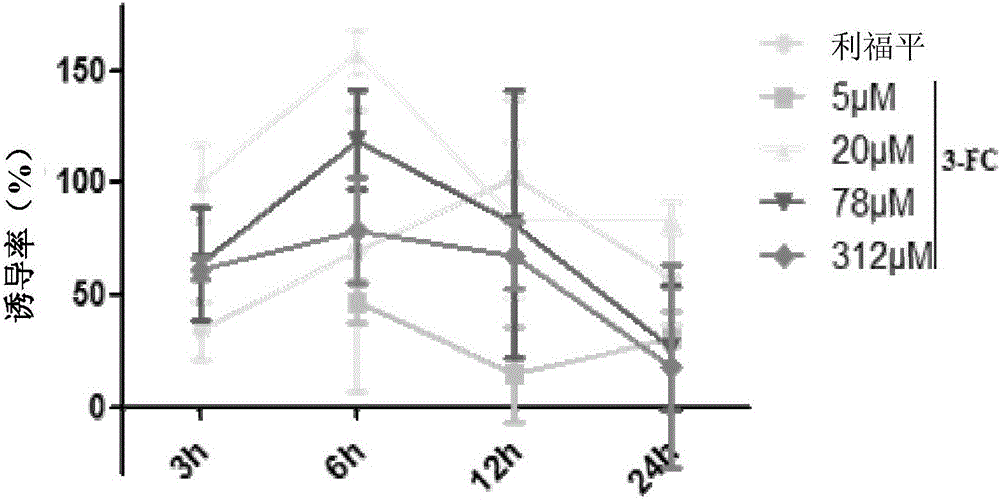
[0046] Example 3, Test of Induced Effect of Test Drugs on Intracellular Nitric Oxide in Macrophages Infected by Mycobacterium tuberculosis
[0047] Test drug: 3-fluorocatechol, product of Sigma-Aldrich, product number 344656.
[0048] THP-1 cells were divided into 5×10 5 Cells / mL were inoculated in 96-well plates, 100 μL per well, and differentiated with 100 ng / mL phorbol ester (PMA) for 24 hours to adhere to the wall to obtain macrophages derived from THP-1 cells. Macrophages derived from THP-1 cells were infected with Mycobacterium bovis BCG 1173P2 at an infectivity of MOI=10 for 4 hours. Wash away extracellular BCG 1173P2 with PBS, add 199 μL of 1640 medium containing 10% (volume fraction) fetal bovine serum, and then add 1 μL of each test drug at different concentrations, so that the final concentrations in the system are 5 μM, 20 μM, and 78 μM respectively , 312μM, and continue to incubate for 3h, 6h, 12h and 24h, respectively. After drug action at different times, dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com