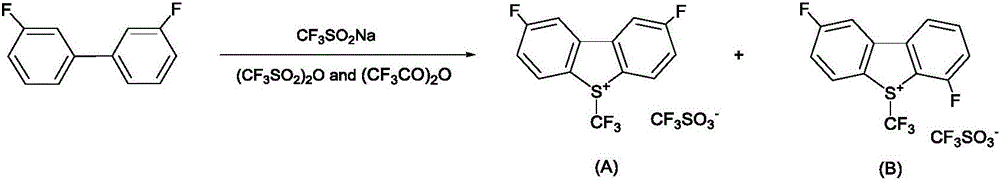
Industrial production method of 3,3'-difluorobiphenyl
A technology of difluorobiphenyl and fluorophenylmagnesium halide, which is applied in the fields of chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problem of no electron-donating property for meta-fluorine substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1. Process I: the preparation of 3,3'-difluorobiphenyl
[0068]
[0069] In a 500ml four-neck flask equipped with a dropping funnel, a thermometer, and a magnetic stirrer, add 200ml of anhydrous tetrahydrofuran and 3.75 grams (154 mmol) of magnesium metal. Add 24.5 g (140 mmol) of 3-fluorobromobenzene in the dropping funnel. A small amount of iodine and a small amount of 3-fluorobromobenzene were added to the reaction flask, and then the reaction mixture was heated to about 50° C. to initiate the Grignard reaction. After the Grignard reaction is initiated, the 3-fluorobromobenzene in the dropping funnel is slowly dropped into the reaction solution to control the internal temperature to 45°C-52°C. After dropping, the reaction solution was stirred for 3 hours until the reaction was complete.
[0070] Add 84ml of dry tetrahydrofuran, 8.3g (83.8mmol) of 1,2-dichloroethane, and 0.7g (4.3mmol) of 3mol% FeCl to another four-necked flask equipped with a thermome...
Embodiment 2
[0071] Embodiment 2. technique II: under the effect of tetrabutylammonium chloride, the product gained in technique I is processed with sodium hydroxide aqueous solution.
[0072] The process I product is obtained by standard post-treatment after process I reaction. The standard aftertreatment is acidification with dilute hydrochloric acid, extraction with ethyl acetate, drying over magnesium sulfate, vacuum distillation after concentration, and the purity of the product 3,3'-difluorobiphenyl obtained in the GC analysis process I is 97.0% (NMR analysis purity is 95 %).
[0073] In a 1L bottle, add 200g process I to prepare the resulting product, 196ml contains 2.5N (mol / L) of sodium hydroxide of the phase-transfer catalyst tetrabutylammonium chloride of 5mol% (relative to sodium hydroxide) aqueous solution. Under nitrogen, the mixture was heated to reflux for 3 hours and then allowed to cool to room temperature. The reaction mixture was acidified with dilute hydrochloric ac...
Embodiment 3-5
[0074]Example 3-5. Process II: Under the action of a phase transfer catalyst, the crude product obtained in Process I is treated with an aqueous alkali or acid solution.
[0075] Like embodiment 2, the used raw material of embodiment 3-5 is the product that process 1 obtains. Under the action of a phase transfer catalyst or not under the action of a phase transfer catalyst, the impurity-containing product obtained in process I is treated with an aqueous alkali or acid solution. Table 1 shows the bases, acids and their amounts, reaction conditions and results. High yield and high purity products can be obtained.
[0076] Table I. Process 1 obtained products are treated with alkaline water, aqueous acid solution or pure water
[0077]
[0078]
[0079] * Purity by NMR analysis. >99% means no impurities detected by NMR.
[0080] ** Purity was analyzed by GC (FID detector).
[0081] + Oil bath temperature is 120°C
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com