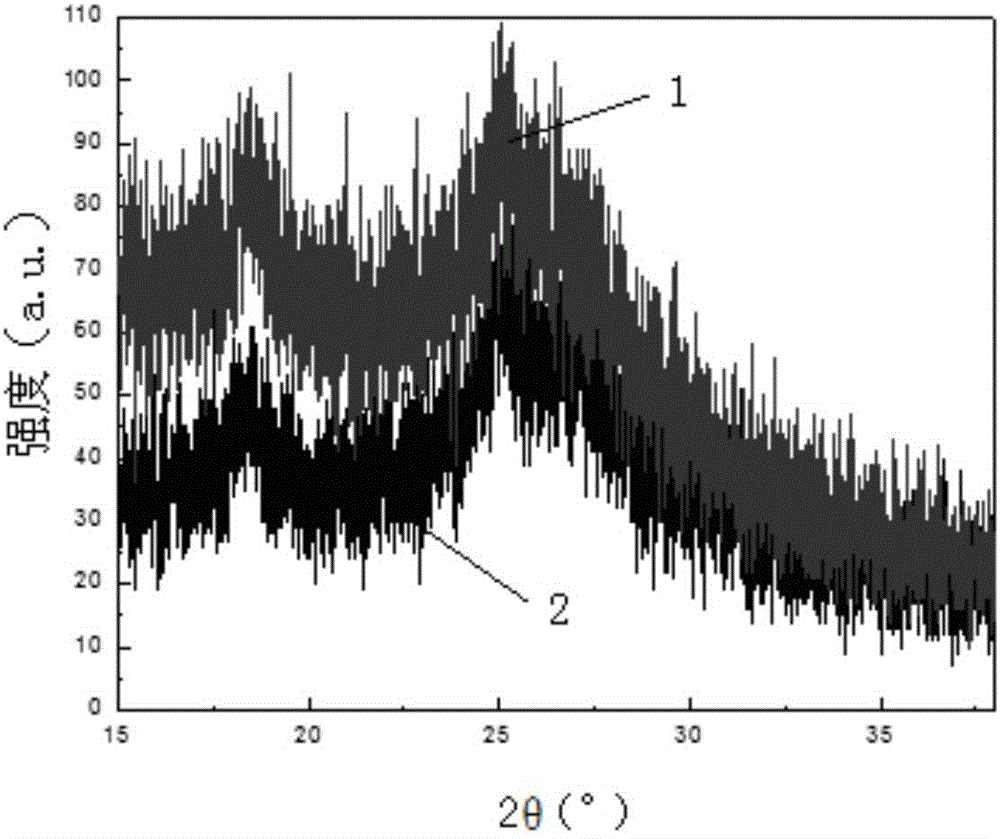
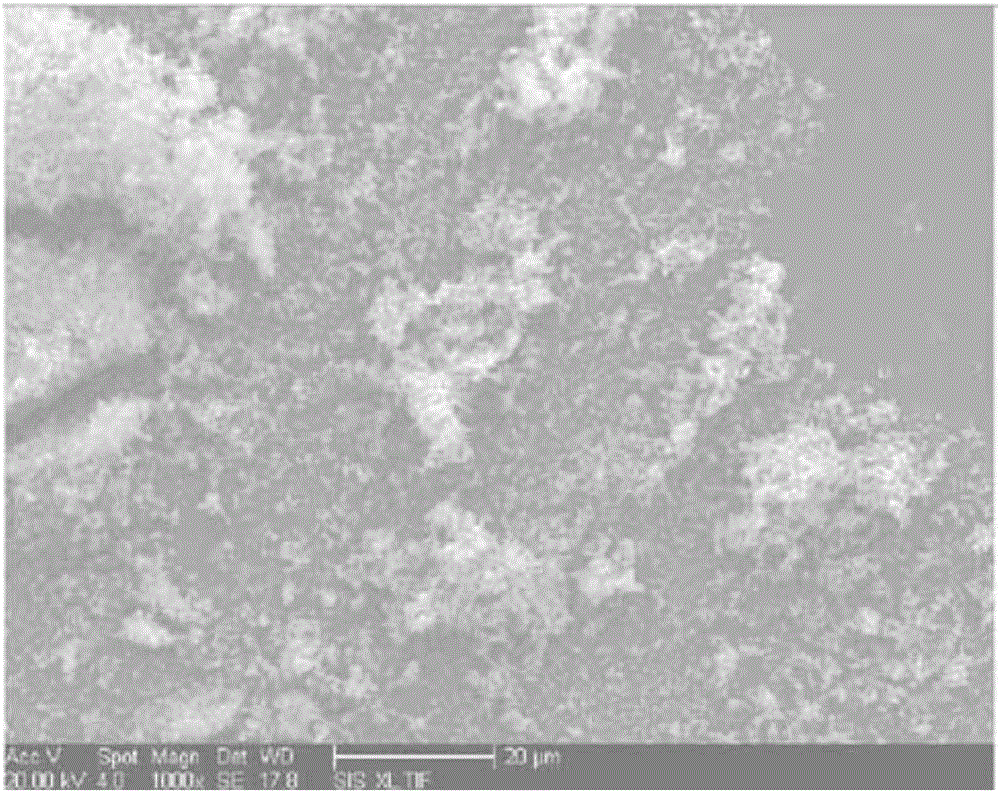
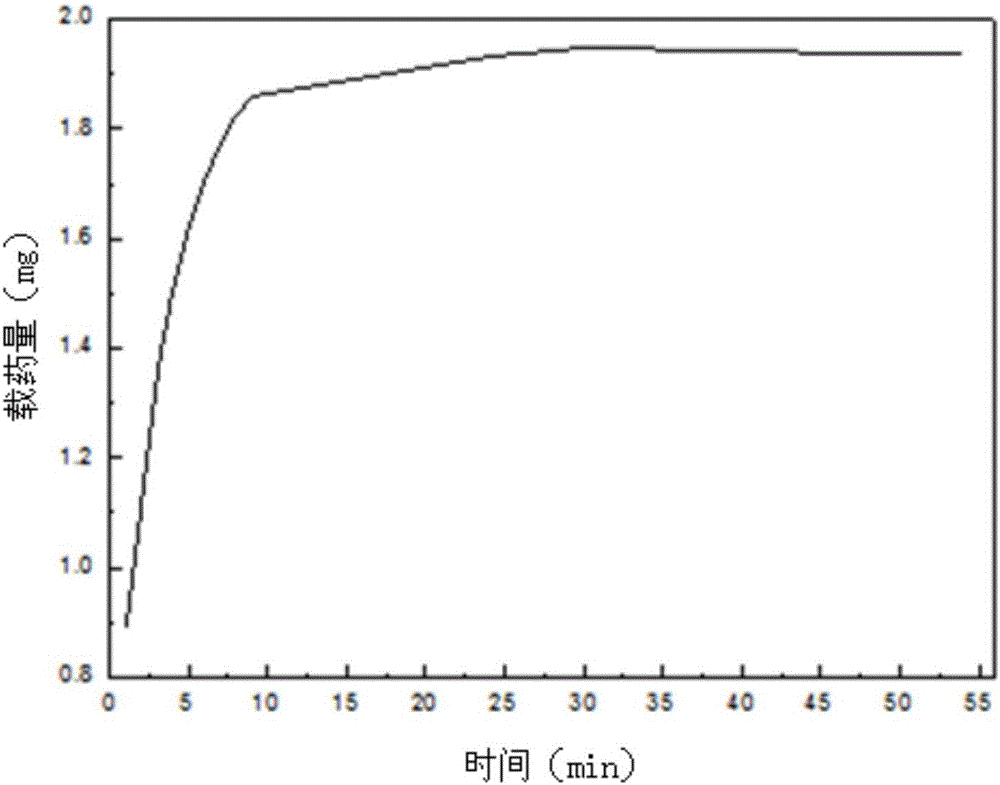
Preparation method of Schiff base two-dimensional polymer drug carrier material
A technology of carrier materials and polymers, which is applied in drug combinations, antineoplastic drugs, organic chemistry, etc., can solve the problem of low bioavailability of 5-fluorouracil, and achieve the effect of increasing bioavailability, low production cost, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0024] Specific embodiment one: the preparation method of a kind of Schiff base two-dimensional polymer drug carrier material of the present embodiment is carried out according to the following steps:
[0025] 1. Preparation of the precursor trihydrazine triazine: disperse cyanuric chloride in absolute ethanol, then add hydrazine hydrate aqueous solution at a rate of 5mL / min to 7mL / min, heat to a temperature of 40-60°C, and place React in a constant temperature water bath at a temperature of 40-60°C for 1.5h-2.5h, then wash and filter with suction to obtain the precursor trihydrazinetriazine;
[0026] The ratio of the quality of cyanuric chloride described in step 1 to the volume of dehydrated alcohol is 9.24g: (20~40) mL;
[0027] The mol ratio of cyanuric chloride and hydrazine hydrate in the hydrazine hydrate aqueous solution described in step 1 is 0.05:(0.4~0.5);
[0028] 2. Preparation of Schiff base two-dimensional polymer drug carrier material: trimethylbenzene, 1,4-di...
specific Embodiment approach 2
[0033] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the ratio of the quality of cyanuric chloride described in step one to the volume of absolute ethanol is 9.24g:30mL. Other steps and parameters are the same as those in the first embodiment.
specific Embodiment approach 3
[0034] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the molar ratio of cyanuric chloride and hydrazine hydrate in the hydrazine hydrate aqueous solution described in step one is 0.05:0.45. Other steps and parameters are the same as those in Embodiment 1 or 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com