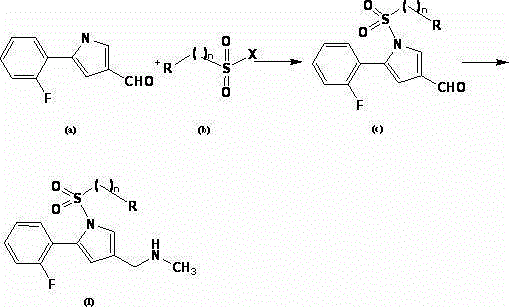
Novel pyrroles derivative as well as preparation method and pharmaceutical application thereof
A technology of pyrrole and pyridine, which is applied in the field of preparation of therapeutic agents and potassium-competitive acid blockers (P-CABs), can solve the problems of slow onset, difficult gastric acid secretion, and large differences in efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
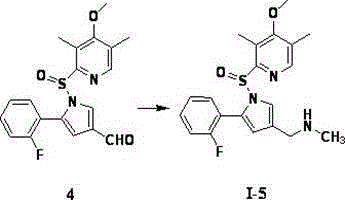
[0046] [5-(2-fluorophenyl)-1-((4-methoxy-3,5-dimethyl-2-pyridine)-3-ylsulfonyl)-1H-pyrrol-3-yl]- N-Methylmethylamine (Ⅰ-1)
[0047]
[0048]
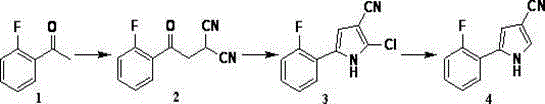
[0049] [2-(2-Fluorophenyl)-2-oxoethyl]malononitrile (2)
[0050] Dissolve 2-fluoroacetophenone (10.0g, 72.4mmol) in ethyl acetate (60ml), then add bromine (12.5g, 78.2mmol) in ethyl acetate (25ml) dropwise, and stir at room temperature for 2 hour, cool down to 0~5°C in an ice bath, slowly add aqueous sodium sulfite solution, continue to stir at room temperature for 1 hour, let stand to separate layers, wash the ethyl acetate layer with water (30ml×2), and then wash with saturated sodium bicarbonate solution (30ml) , and the ethyl acetate layer was collected. The ethyl acetate layer was added to the reaction flask, then malononitrile (4.93g, 74.6mmol) was added, the temperature was cooled to 0~5°C in an ice bath, triethylamine (8.28g, 81.8mmol) was slowly added dropwise, and stirred at room temperature for 3 Hour. Add 50ml of wat...
Embodiment 2
[0071] [5-(2-fluorophenyl)-1-((3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridine)-3-ylsulfonyl)-1H- Pyrrol-3-yl]-N-methylmethylamine (Ⅰ-2)
[0072] The specific operation is the same as that in Compound Example 1, compound (5) is added with 3-methyl-4-(2,2,2-trifluoroethoxypyridine)-2-sulfonyl chloride (6b) to obtain compound (I-2) Pale yellow solid.
[0073] MS (ESI) m / z: 458.1 (M+H) +
Embodiment 3
[0075] [5-(2-fluorophenyl)-1-((3,4-dimethoxy-2-pyridine)-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylformazan Amine (Ⅰ-3)
[0076] The specific operation is the same as that of Compound Example 1. Compound (5) is added with 3,4-dimethoxy-pyridine-2-sulfonyl chloride (6c) to obtain compound (I-3) as a pale yellow solid.
[0077] MS (ESI) m / z: 406.1 (M+H) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com