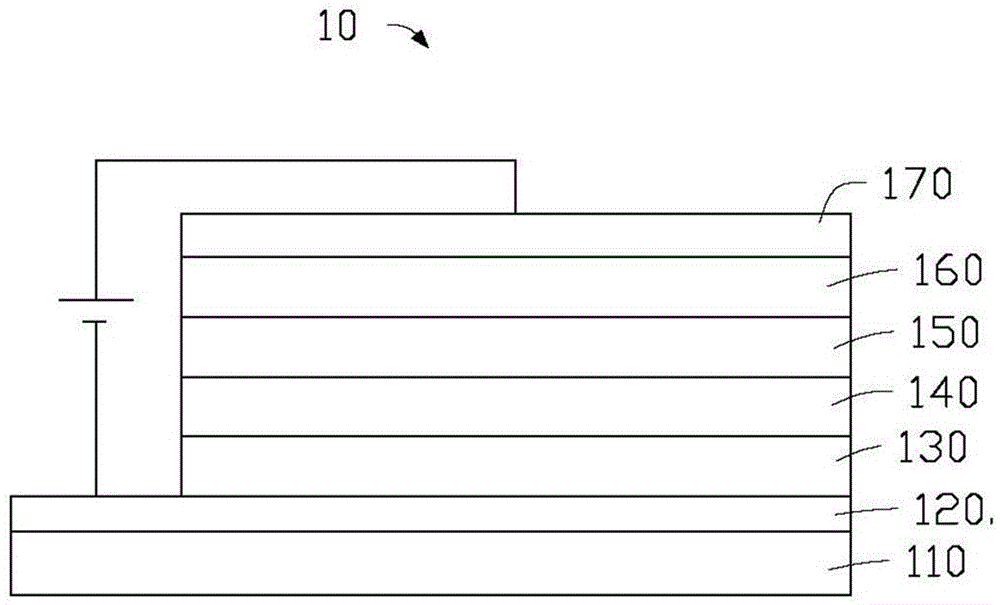
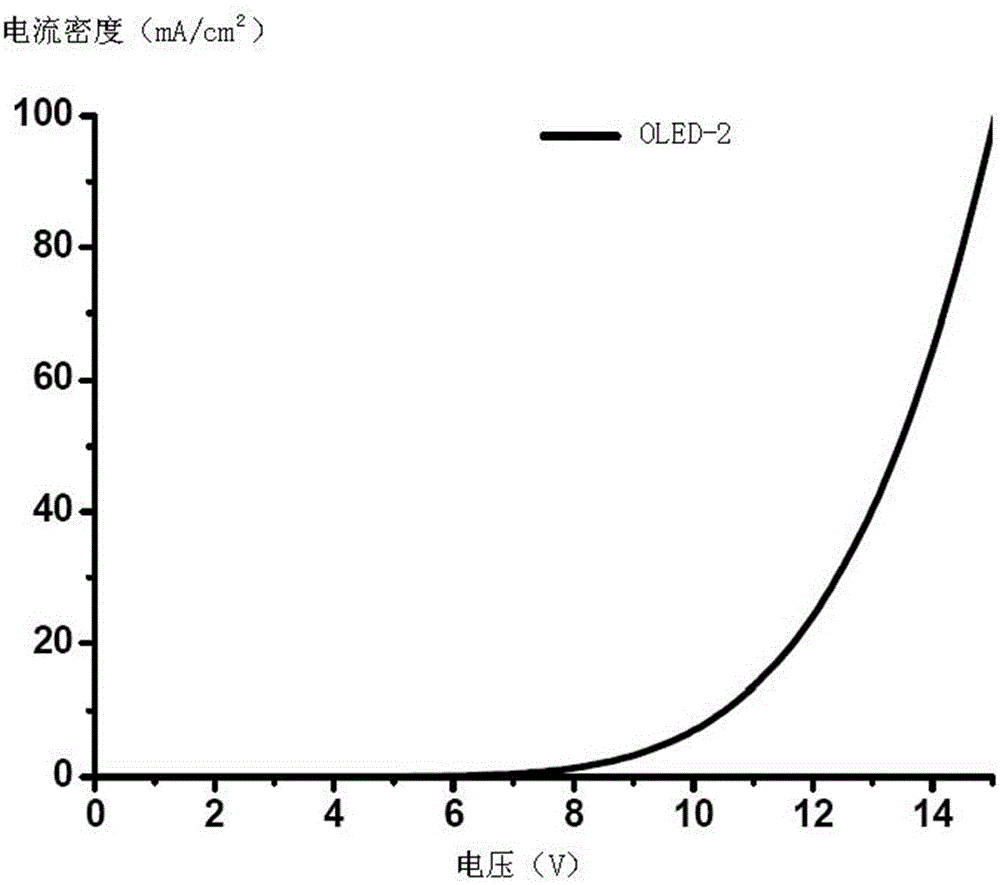
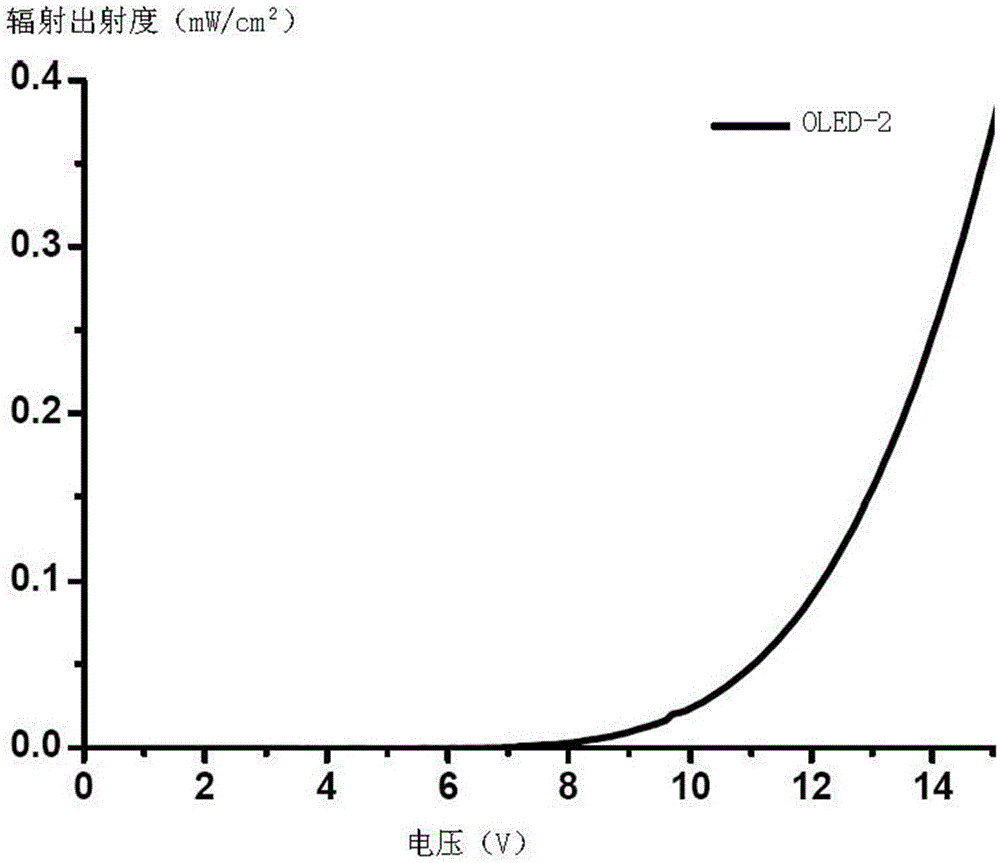
Iridium complex and organic electroluminescence device
A technology of iridium complexes and organic light-emitting layers, applied in the direction of electrical solid devices, electrical components, luminescent materials, etc., can solve the difficulties in the synthesis and purification of target complexes, increase the difficulty of synthesis and purification, and weaken the coordination ability of ligands and other issues, to achieve the effect of increasing interaction, reducing the influence of steric hindrance effect, and overcoming efficiency roll-off
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] The preparation method of the L ligand in the iridium complex is as follows The preparation method of is illustrated as an example. Other L ligands can be obtained by changing the diphenyl iodate with substituents and the cyano groups with the same or different substituents added twice, and will not be repeated here.
[0102] When the L ligand is , the L ligand can be prepared according to the following route:
[0103]
[0104] Specific process: 2 mmol (mmol) Ph 2 IPF 6 , 2mmol thiophenecarbonitrile and 0.4mmol Cu(OTf) 2 Soluble in 1,2-dichloroethane, N 2 Heating to reflux at 120°C for 2h (hour) under (nitrogen) atmosphere, then cooling to room temperature, adding 2 times the equivalent (ie 4 mmol) of thiophenecarbonitrile, heating to reflux at 120°C for 12h, after the reaction, cool the reaction solution to room temperature, add Anhydrous K 2 CO 3 , the organic phase was extracted with dichloromethane, anhydrous Na 2 SO 4 Dry the organic phase, filter, e...
Embodiment 1
[0106] Embodiment 1: the preparation of iridium complex C1
[0107] The iridium complex C1 can be prepared according to the following route:
[0108]
[0109] The specific process is: take 1.472g (5.0mmol) of the main ligand L and 0.975g (2.0mmol) of iridium trichloride, add 40mL of ethylene glycol methyl ether and ionized water with a volume ratio of 3:1 as solvent, and protect Heat it in an oil bath to 110°C under reflux and stir for 24h. After the reaction, the reaction solution was cooled to room temperature, filtered with suction, and the filter cake was collected. After the filtrate was discarded, the filter cake was rinsed with ethanol and a large amount of ether successively. Finally, dichloromethane was used to dissolve the filter cake, the filtrate was collected, the solvent was removed by rotary evaporation, and the mixture was dried in vacuum at 60°C for 5 hours to finally obtain 0.98 g of dark red solid with a yield of about 60%. The ESI-MS (electrospray ion...
Embodiment 2
[0114] Embodiment 2: the preparation of iridium complex C2
[0115] This example is basically the same as Example 1, except that sodium tetrakis(3,5-bis(p-trifluoromethylphenyl))borate was used instead of ammonium hexafluorophosphate, and the product yield was 35%.
[0116] ESI-MS (electrospray ionization mass spectrometry) [m / z]: 935 [M-BArF 24 ] + .
[0117] Elemental analysis (C 58 h 29 BF 24 IrN 4 S 2 ): Anal. Calcd (theoretical): C, 49.42; H, 2.13; N, 4.67; Found (measured): C, 49.53; H, 2.21; N, 4.71.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com