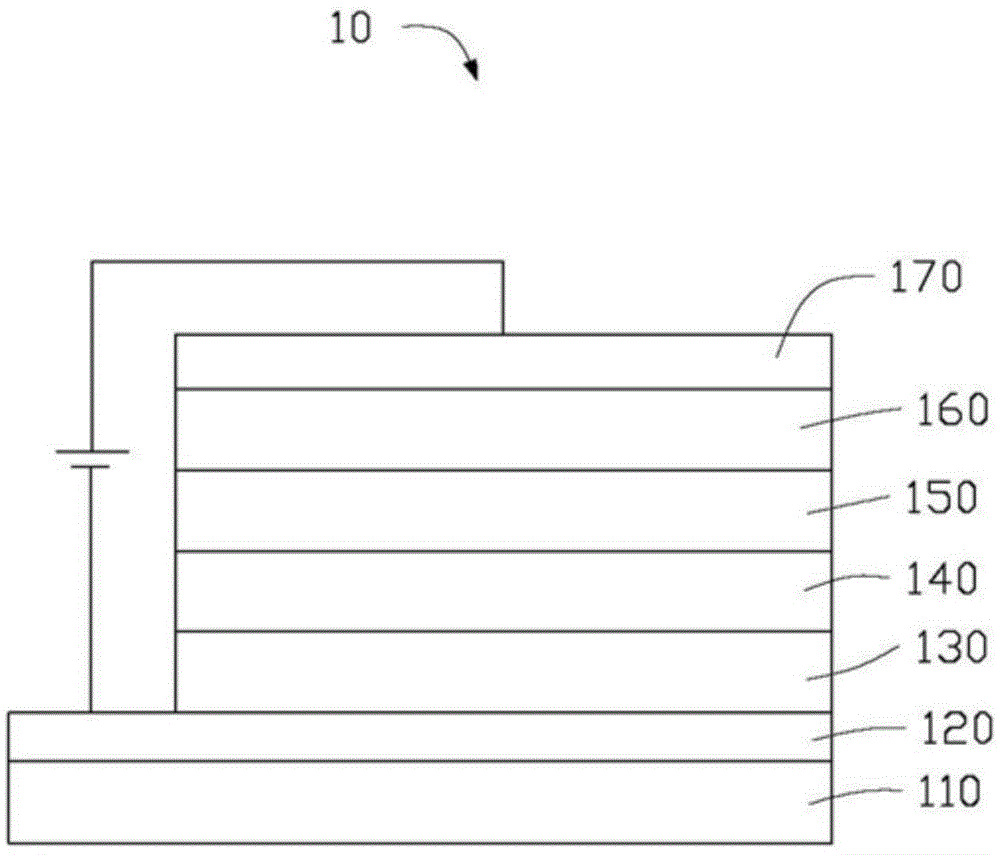
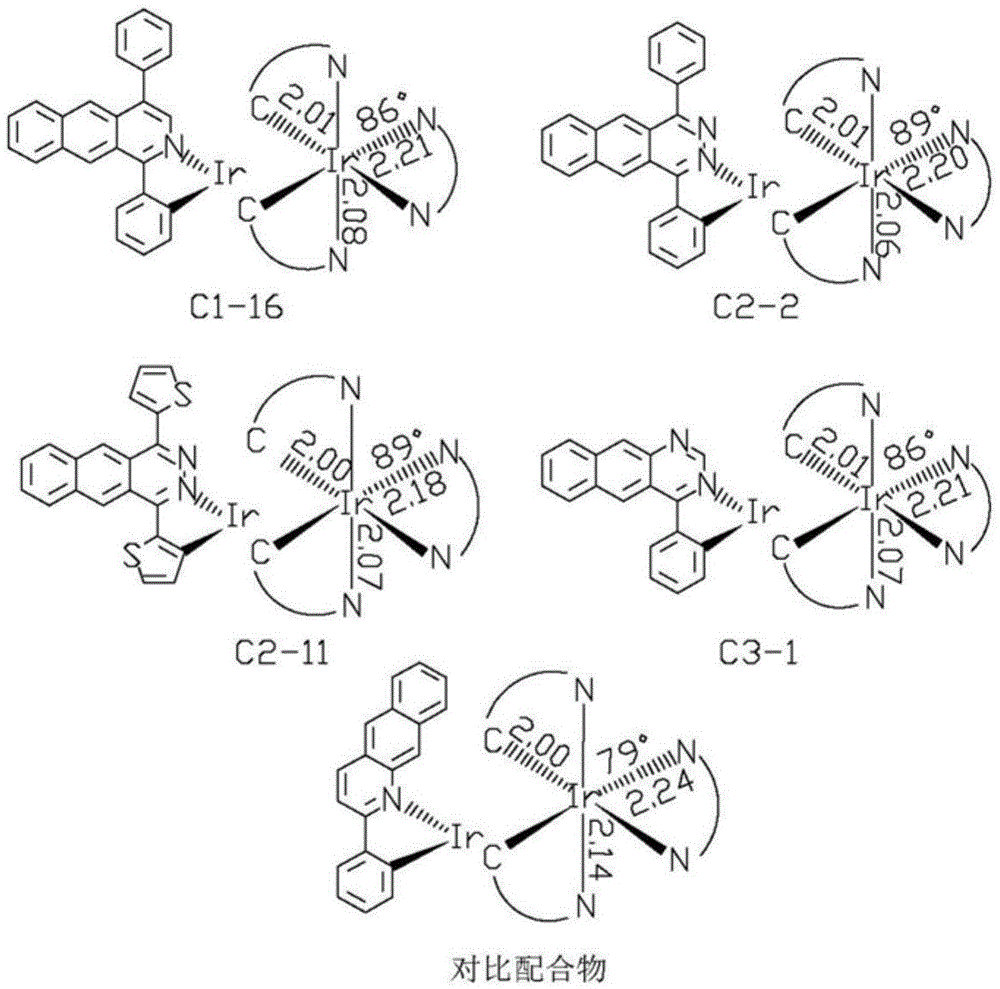
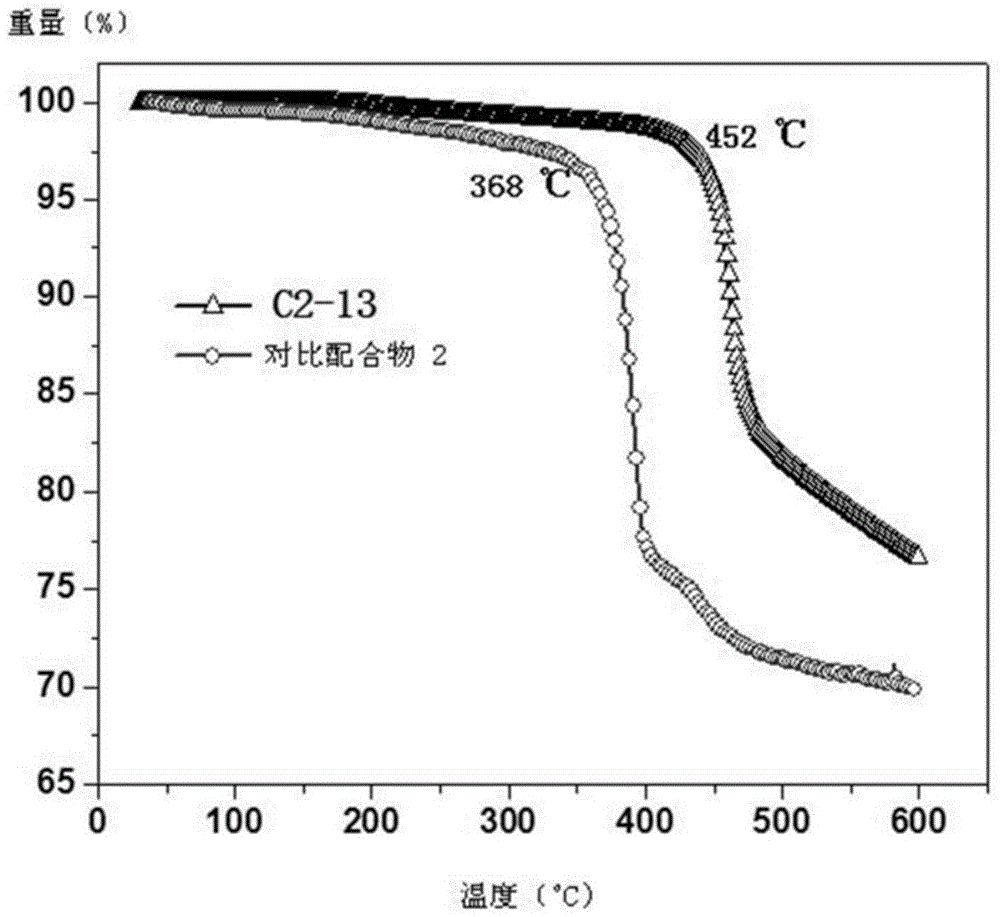
Near-infrared luminous material and OLEDs (organic light-emitting diodes)
A luminescent material and near-infrared technology, applied in luminescent materials, electric solid devices, electrical components, etc., can solve problems such as enlargement and decrease in luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation of the L2 ligand can refer to the following routes and methods:
[0041]
[0042] Dissolve 2.1 g (6.35 mmol) of the ligand raw material in 40 mL of methanol, add excess hydrazine hydrate, stir at room temperature for 1 hour until yellow precipitates, and then recrystallize with methanol, yield 90%.
[0043] Different said L2 ligands can be obtained with substituted formyl ethane and hydrazine hydrate.
[0044] The preparation of the L3 ligand can refer to the following routes and methods:
[0045]
[0046] Dissolve 2mmol of 3-aminonaphthalene-2-benzophenone, 3mmol of glycine, 1mmol of iodine and 0.55mL of tert-butyl hydroperoxide (70% aqueous solution) in 5mL of dimethylformamide, and place in a sealed tube at 80°C React for 18 hours. Separated by column chromatography, a yellow solid was obtained with a yield of 70%.
[0047] Different L3 ligands can be obtained with substituted 3-aminonaphthalen-2-benzophenones and substituted glycine.
[004...
Embodiment 1
[0059] Embodiment 1: Preparation of iridium complex C1-1
[0060]
[0061] IrCl 3 ·xH 2 O (58% Ir) and 2.2 times the chemical equivalent of L1 ligand were dissolved in a mixed solvent of ethylene glycol methyl ether and deionized water (v / v=3 / 1). The mixture was stirred under reflux at 110° C. for 24 hours under an Ar atmosphere. After cooling to room temperature, filter, wash the filter cake with deionized water until neutral, then rinse the filter cake with 10mL ethanol and 200mL diethyl ether in sequence. Finally, the filter cake was dissolved with dichloromethane, the filtrate was collected, the solvent was evaporated, and vacuum-dried at 70° C. for 5 hours to obtain the dichloro-bridged intermediate as a dark brown solid with a yield of 85%, which was directly put into the next reaction without further purification.
[0062] Dissolve 0.34mmol of the dichloro-bridged intermediate and 1.0g of 4,7-diphenyl-1,10-phenanthroline in 40mL of ethylene glycol, protect the rea...
Embodiment 2
[0065] Embodiment 2: the preparation of iridium complex C1-2
[0066]
[0067] Please refer to Example 1 for the preparation method of the dichloro-bridged intermediate.
[0068] 0.17mmol of dichloro-bridged intermediate, 0.05g (0.5mmol) of acetylacetone and 0.056g (0.5mmol) of potassium tert-butoxide were dissolved in 12mL of dichloromethane / ethanol (volume ratio=3 / 1) mixed solvent. The reaction system was protected by argon, and stirred under reflux at 30°C for 24 hours. After cooling to room temperature, the solvent was evaporated to dryness, then dissolved in dichloromethane, extracted three times with deionized water, and washed with anhydrous MgSO 4 The organic phase is dried. Filter, distill off the solvent, separate by column chromatography, and collect the black product band. After concentration, it was recrystallized with dichloromethane / ether to obtain a black solid with a yield of 45%.
[0069] 1 H-NMR (nuclear magnetic resonance, CDCl 3 ,300MHz,δ[ppm]): 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com