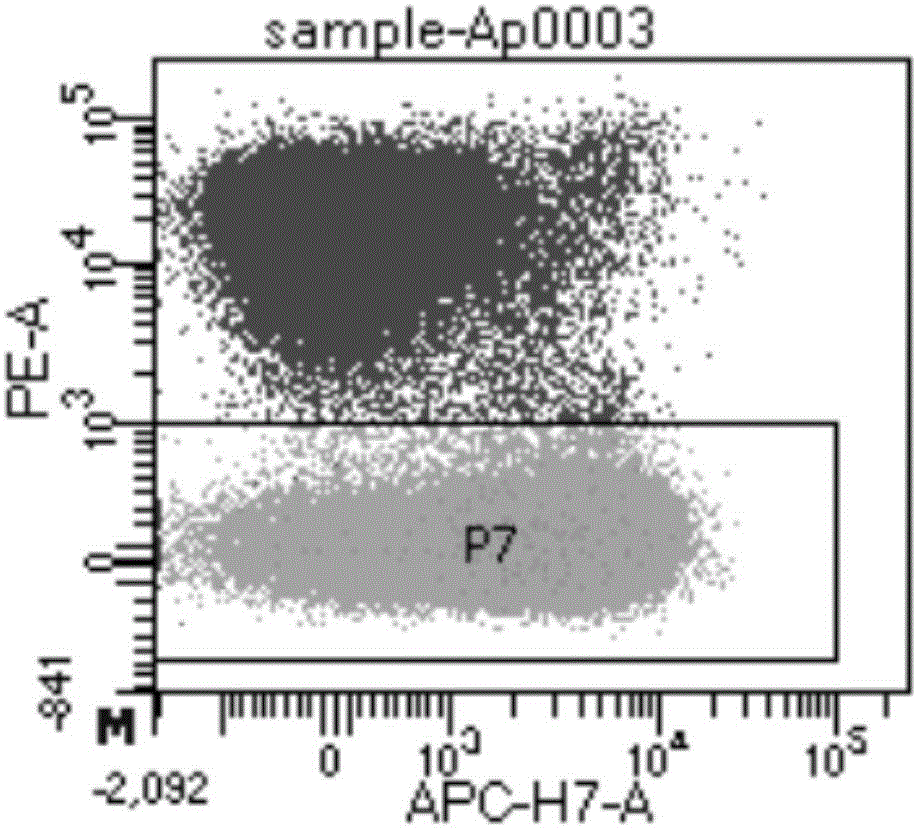
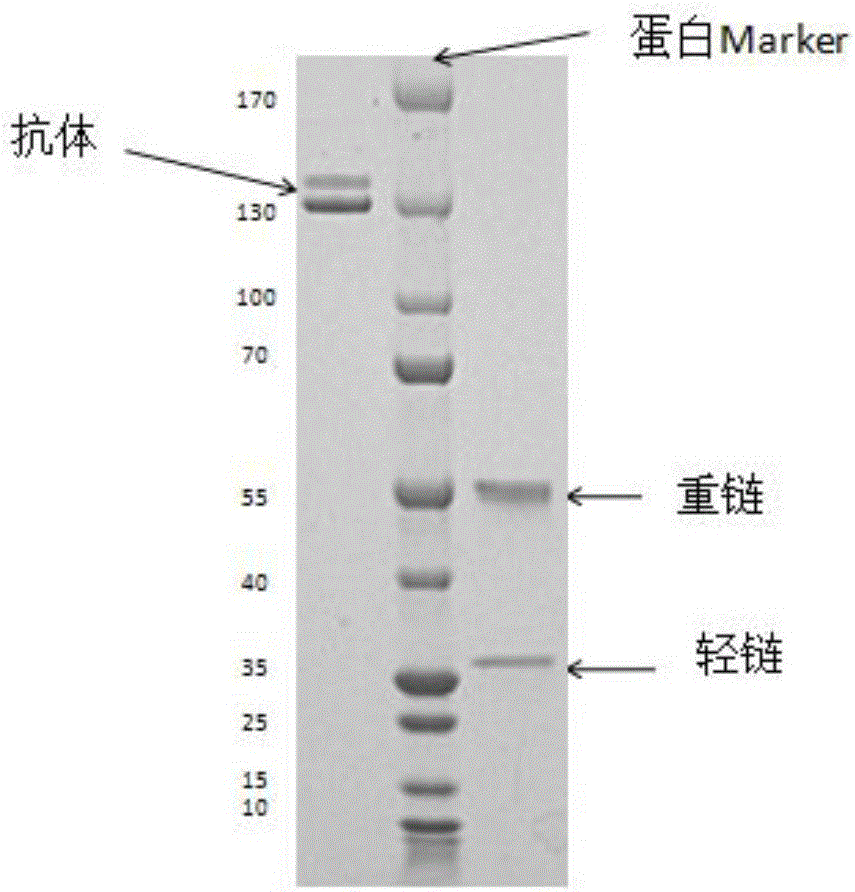
Fully-humanized rabies virus resisting neutralizing antibody
A rabies virus, fully human technology, applied in the fields of genetic engineering and cellular immunology, can solve problems such as failure to complete preclinical research, complex antibody process, chimeric antibodies and humanized antibodies can not completely solve the HAMA reaction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Lymphocyte Separation and Single Memory B Cell Sorting
[0060] 1. Materials:
[0061] 1. Reagent: rabies vaccine (Novartis, batch number: 1928). Ficoll lymphocyte separation medium is a product of Cedarlane Company. CD3-APC, CD14-APC, CD16-PE, CD20-APC, CD27-PE, IgD-FITC, etc. are all products of Invirogen.
[0062] 2. Method results
[0063] 1. Volunteer immunization with rabies vaccine
[0064] Two 25-year-old healthy volunteers who were inoculated with rabies vaccine were selected, and they received booster vaccinations on the 7th day and 21st day respectively, and the quantitative detection of 28d IgG was positive; 100 mL of their peripheral blood was drawn for anticoagulation.
[0065] 2. Lymphocyte isolation and single memory B cell sorting
[0066] Separate 100mL blood sample with Ficoll, absorb the mononuclear cell (PBMC) layer suspension, wash with PBS 3 times, and discard the supernatant. Set single dye tube: add AqVd-AmCyan, CD3-PE-Cy5, IgD-P...
Embodiment 2
[0069] Example 2 Single B cell RT-PCR isolation antibody variable region gene
[0070] 1. Materials
[0071] 1. Reagents: primers (synthesized by Invitrogen), Superscript III reverse transcriptase, HotStarTaq Plus enzyme (Invitrogen, Carlsbad, CA), etc.
[0072] 2. Method results
[0073] 1. Single B cell RT-PCR (synthesis of the first strand of cDNA)
[0074] Add 0.5uM constant region primers of heavy and light chains of each subtype to a 96-well plate containing a single B cell and Superscript III reverse transcriptase, and incubate at 37°C for 1 hour; carry out PCR amplification under the following conditions: 95°C for 15min ; 95°C for 1min, 55°C for 1min, 72°C for 1min, 30 cycles; 72°C for 10min; 4°C for 5min. The product cDNA was stored at -20°C.
[0075] 2. Nest-PCR (isolation of antibody gene)
[0076] Each single cell amplifies the heavy chain and light chain sequences separately. The 50uL system contains 5uL of RT reaction products, HotStarTaq Plus enzyme, dNTPs...
Embodiment 3
[0077] Example 3PCR product clone identification and antibody expression
[0078] 1. Materials
[0079] 1. Reagents: Agarose, Tris, LB, Ampicillin, PolyFect (Qiagen, Valencia, CA), FCS, DMEM, PBS, etc.
[0080] 2. Vector: pcDNA3.3 vector
[0081] 3. Strain: Escherichia coli DH5α
[0082] 2. Method results
[0083] 1.PCR product purification clone identification
[0084] Take 2 μL of the amplification product and detect it by 1.2% agarose gel electrophoresis, and the target fragment is about 400 bp. The gel electrophoresis was identified as positive, and the PCR product of the antibody variable region gene whose heavy chain and light chain could be paired was connected to the pcDNA3.3 vector (which has been transformed and contains the antibody leader and constant region) by the method of TA cloning, Transform the ligation product into DH5α-competent bacteria, culture on a plate containing ampicillin at 37°C overnight, then pick 10 single colonies and perform PCR with spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com