Fully human anti-rabies virus neutralizing antibody and application thereof
A rabies virus, all-human technology, applied in the field of cellular immunology and molecular biology, can solve the problem of no drug approval for marketing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of fully human anti-rabies virus monoclonal neutralizing antibody TRN073
[0071] 1. Memory B cell sorting
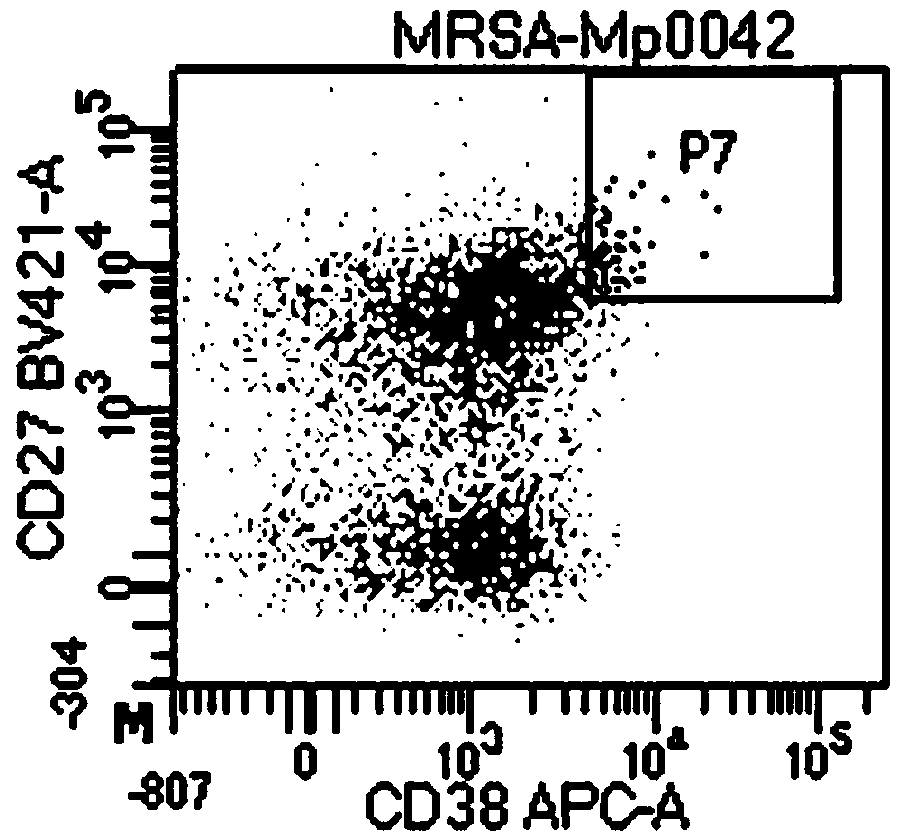
[0072] Two healthy volunteers were selected to be injected with rabies vaccine (Novartis, batch number: 1928). On the 7th day (28th day) after the full immunization, 100 mL of blood was drawn on an empty stomach. ELISA was used to detect positive results for rabies virus IgG. Then, the anticoagulated whole blood samples were separated with Ficoll lymphocyte separation medium to obtain human peripheral blood mononuclear cells (PBMC), and the CD3-, CD14-, CD16-, CD19+, CD27+ single B cells, the results are as follows figure 1 shown. A single B cell obtained by sorting was placed in a 96-well PCR plate containing an RT reaction system so that each well contained one memory B cell, and the PCR plate was frozen at -80°C for future use.
[0073] 2. Reverse transcription to synthesize cDNA strand
[0074] Add 0.5 μM constant region primers and ...
Embodiment 2
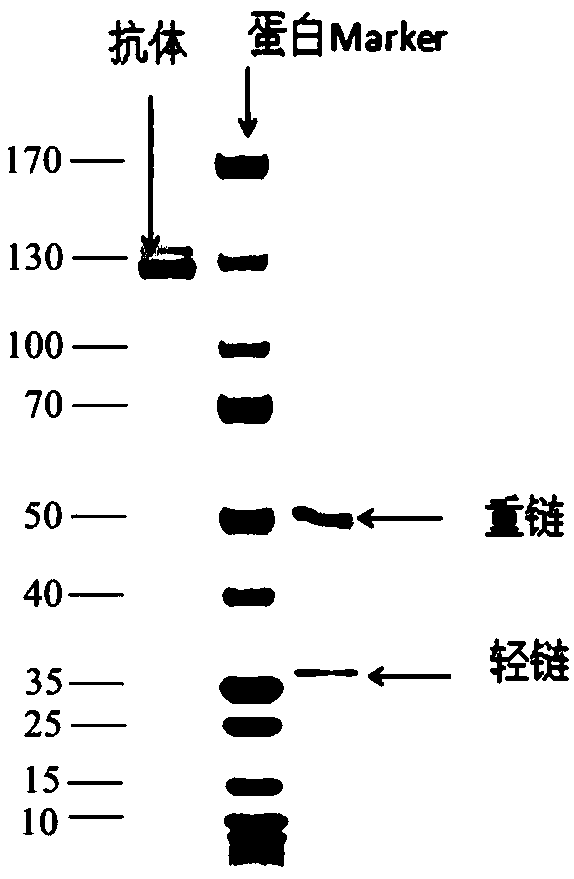
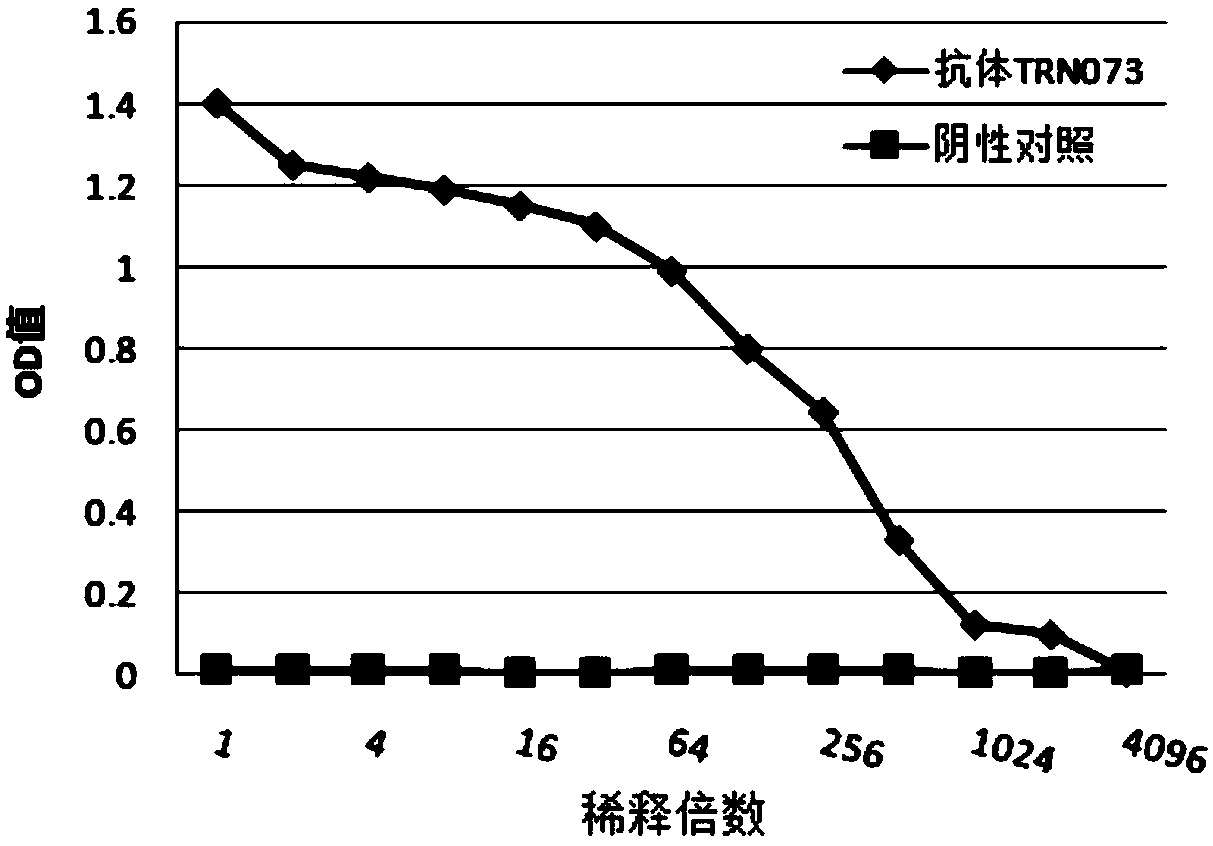
[0085] Example 2 TRN073 Antibody Binding Activity Detection
[0086] Use the same ELISA method mentioned above to detect the binding activity of the expressed and purified antibody: use rabies vaccine as the antigen, and coat the ELISA 96-well plate after diluting the antigen 10 times with the coating solution, and coat each well with 100 μL overnight at 4°C , and blocked with blocking solution for 2 hours at room temperature. Dilute the TRN073 antibody supernatant with an initial concentration of 100 μg / ml 10 times and then use it as the primary antibody for serial dilution. Add the primary antibody and incubate at room temperature for 2 hours. At the same time, use the untransfected cell supernatant as a negative antibody control. Goat anti-human IgG (diluted at 1:2000) was incubated as the secondary antibody at room temperature for 1 hour, and 100 μl / well of the substrate chromogenic solution was added. After standing in the dark at room temperature for 5 minutes, the react...
Embodiment 3
[0088] Example 3 TRN073 Antibody Binding Specific Detection
[0089] (1) Binding to rabies virus G protein (RVG)
[0090] Steps: It is the main protective antigen of rabies virus, which can stimulate the body to produce neutralizing antibodies, clone the gene fragment of RVG into the eukaryotic expression vector pcDNA3.1, and transfect 293T with PolyFect (Qiagen, Valencia, CA) transfection reagent cells, take 1×10 5 48-72 hours after transfection, centrifuge 293T cells at 300×g for 10 minutes, remove the supernatant, add 10 μL of commercial human anti-rabies virus serum or antibody TRN073, and use other expressed human monoclonal antibodies as negative controls, and stain at 4°C After incubation for 45 minutes, wash twice with PBS, then add FITC-labeled goat anti-human IgG antibody, stain and incubate at 4°C for 30 minutes, wash twice with PBS, and analyze with BD FACSria flow cytometer.
[0091] The results indicated that antibody TRN073 is a functional antibody specific to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com