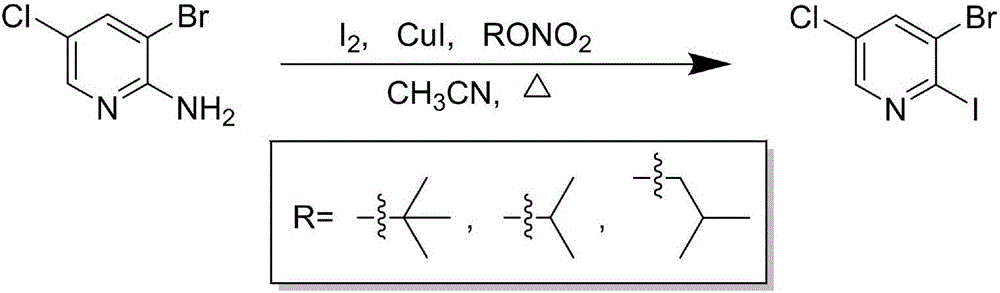
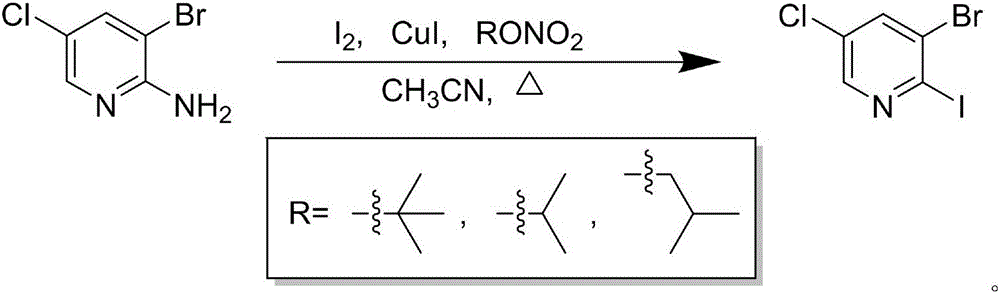
Preparation process of 2-iodo-3-bromo-5-chloropyridine
A technology of chloropyridine and elemental iodine, applied in the field of preparation of 2-iodo-3-bromo-5-chloropyridine, can solve the problems of harsh reaction conditions, many by-products, troublesome post-processing operations, etc., and achieves mild reaction conditions, Environmentally friendly, cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Under nitrogen protection, acetonitrile (100mL), elemental iodine (44.0g, 173.5mmol), cuprous iodide (18.4g, 96.4mmol) and tert-butyl nitrite (13.9g, 135.0mmol) were successively added into a 500mL three-necked flask , and then slowly added 2-amino-3-bromo-5-chloropyridine (20.0 g, 96.4 mmol) under ice bath, and reacted at 60° C. for 2 h. After completion of the reaction, add water (45mL), filter with suction, rinse the filter cake with ethyl acetate (45mL×1), extract the filtrate with ethyl acetate (200mL×2), combine the organic phases, and wash with saturated aqueous sodium thiosulfate ( 100mL×2), washed with anhydrous sodium sulfate, evaporated to remove the solvent under reduced pressure, the residue was slurried with a small amount of methanol, filtered with suction, and the filter cake was dried to obtain 21.5g of 2-iodo-3-bromo-5-chloropyridine as a white solid , the yield was 70.0%.
[0013] 1 H-NMR (CDCl 3 , 400MHz,), δ: 7.835(d,1H), 8.304(d,1H); MS, m / z: 32...
Embodiment 2
[0015] Under nitrogen protection, acetonitrile (100mL), elemental iodine (48.9g, 192.8mmol), cuprous iodide (18.4g, 96.4mmol) and tert-butyl nitrite (14.9g, 144.6mmol) were successively added into a 500mL three-necked flask , and then slowly added 2-amino-3-bromo-5-chloropyridine (20.0 g, 96.4 mmol) under ice bath, and reacted at 60° C. for 2 h. After completion of the reaction, add water (45mL), filter with suction, rinse the filter cake with ethyl acetate (45mL×1), extract the filtrate with ethyl acetate (200mL×2), combine the organic phases, and wash with saturated aqueous sodium thiosulfate ( 110mL×2), washed with anhydrous sodium sulfate, evaporated to remove the solvent under reduced pressure, the residue was slurried with a small amount of methanol, filtered with suction, and the filter cake was dried to obtain 21.4g of 2-iodo-3-bromo-5-chloropyridine as a white solid , the yield was 69.6%.
[0016] 1 H-NMR (CDCl 3, 400MHz,), δ: 7.835(d,1H), 8.304(d,1H); MS, m / z: 323...
Embodiment 3
[0018] Under nitrogen protection, acetonitrile (100mL), elemental iodine (53.8g, 212.1mmol), cuprous iodide (20.2g, 106.0mmol) and tert-butyl nitrite (13.9g, 135.0mmol) were successively added into a 500mL three-necked flask , and then slowly added 2-amino-3-bromo-5-chloropyridine (20.0 g, 96.4 mmol) under ice bath, and reacted at 60° C. for 2 h. After completion of the reaction, add water (45mL), filter with suction, rinse the filter cake with ethyl acetate (45mL×1), extract the filtrate with ethyl acetate (200mL×2), combine the organic phases, and wash with saturated aqueous sodium thiosulfate ( 110mL×2), washed with anhydrous sodium sulfate, evaporated to remove the solvent under reduced pressure, the residue was slurried with a small amount of methanol, filtered with suction, and the filter cake was dried to obtain 21.6g of 2-iodo-3-bromo-5-chloropyridine as a white solid , and the yield was 70.3%.
[0019] 1 H-NMR (CDCl 3, 400MHz,), δ: 7.835(d,1H), 8.304(d,1H); MS, m / z...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com