Test method for evaluating in-vitro antibacterial activity of antibacterial substance
A technology of antibacterial activity and antibacterial substances, applied in the field of biological control, can solve the problems of special properties of traditional Chinese medicine extracts, affecting the measurement of antibacterial zone, dark and turbid color, etc., and achieve the effect of intuitive and rapid antibacterial effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
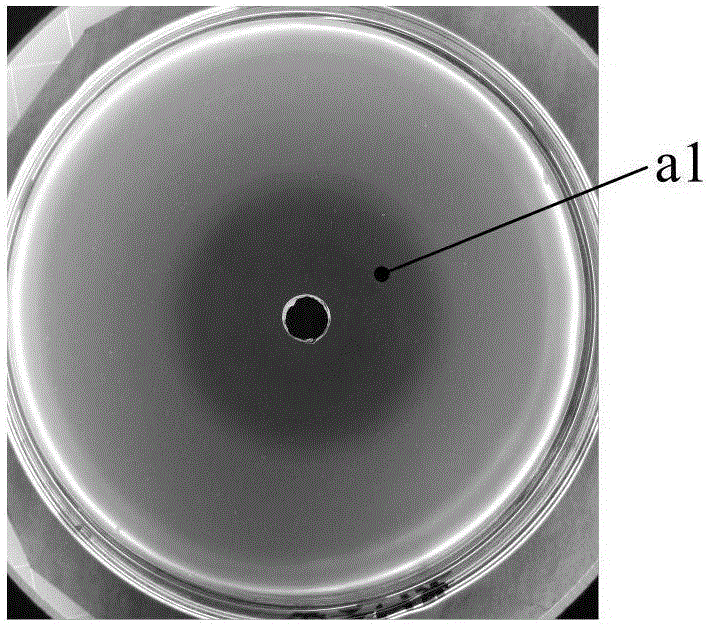
[0030] 1. Preparation of methylene blue agar plates
[0031] 1.1 Preparation of indicator bacteria suspension: Inoculate Salmonella on ordinary slant medium to activate and grow for 6-8 hours. Gently scrape the bacterial lawn on the slope with an inoculation loop and place it in sterile physiological saline, vortex shaker to fully shake and adjust the concentration of the bacterial suspension to 0.5 McFarland turbidity.
[0032] 1.2 Preparation of bacteria-containing methylene blue agar plate: Weigh methylene blue and add distilled water to prepare a 1% methylene blue aqueous solution, which is sterilized for use. After the M-H agar is sterilized in the Erlenmeyer flask, the water bath is kept at a constant temperature of 45°C. Add sterilized 1% methylene blue aqueous solution according to the ratio of adding 2ml methylene blue aqueous solution to every 100ml of bacteria-containing methylene blue agar. Add 0.5 McFarland turbidity bacterial suspension into 6ml of bacterial liq...
Embodiment 2
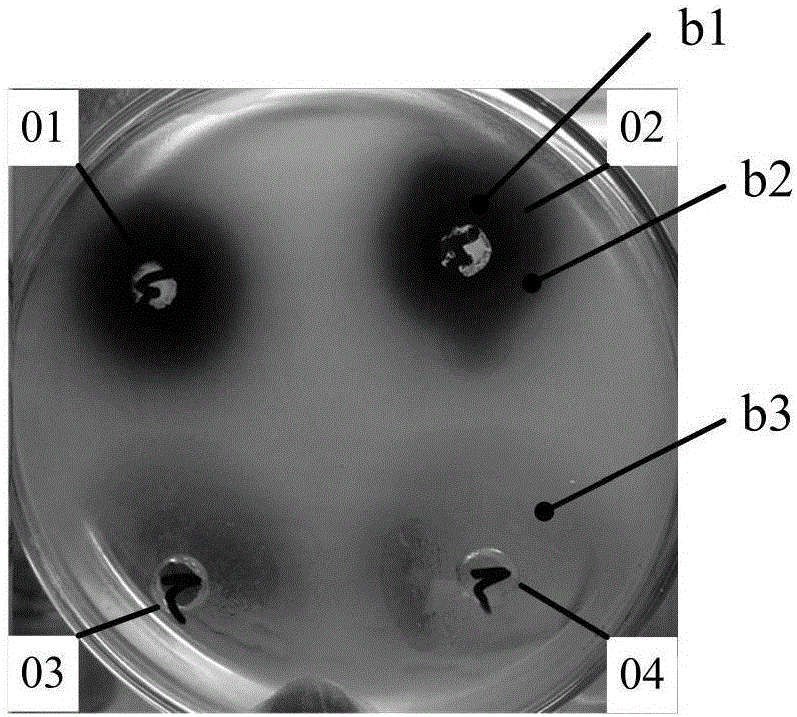
[0045] Application of methylene blue bacteria-containing agar plate diffusion method in external antibacterial test of traditional Chinese medicine extraction liquid
[0046] Test procedure: 1, the preparation method of the methylene blue bacteria-containing agar plate is the same as that of Example 1, and the strains are respectively 2 kinds of Salmonella enteritidis and Salmonella typhimurium;
[0047] 2. Use a puncher with a diameter of 6mm to punch holes on the methylene blue bacteria-containing agar plate, and add the Chinese medicine water extract into the holes for diffusion. The Chinese medicine water extract is respectively taken from the water extract of Schizandra chinensis and the extract of black plum, and the concentration is 1g. / mL.
[0048] In vitro antibacterial effect evaluation: After the plate was incubated at a constant temperature of 37°C for 18 hours, the diameter of the blue circle or the mixed color circle of the drug itself and the blue color on the ...
Embodiment 3
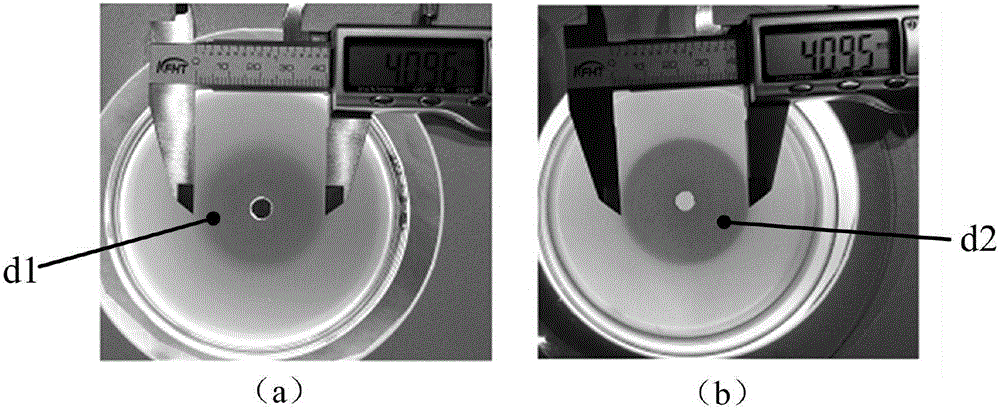
[0052] Antibacterial test of different concentrations of Scutellaria baicalensis water extract on methylene blue bacteria-containing agar plate diffusion method
[0053] Test procedure: 1, the methylene blue bacteria-containing agar plate preparation method is the same as in Example 1;
[0054] 2. Use a hole puncher with a diameter of 6mm to make holes on the methylene blue bacteria-containing agar plate, and add the water extract of Scutellaria baicalensis into the holes for diffusion. mL.
[0055] Evaluation of antibacterial effect in vitro: After the plate was incubated at a constant temperature of 37°C for 18 hours, the diameter of the blue circle or the mixed color circle of the drug itself and the blue color on the methylene blue agar plate was measured to determine the diameter of the inhibition zone and comprehensively evaluate the antibacterial activity.
[0056] Test results: if Figure 6 as shown, Figure 6 -a, 6-b, and 6-c are the results of the interaction betw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com