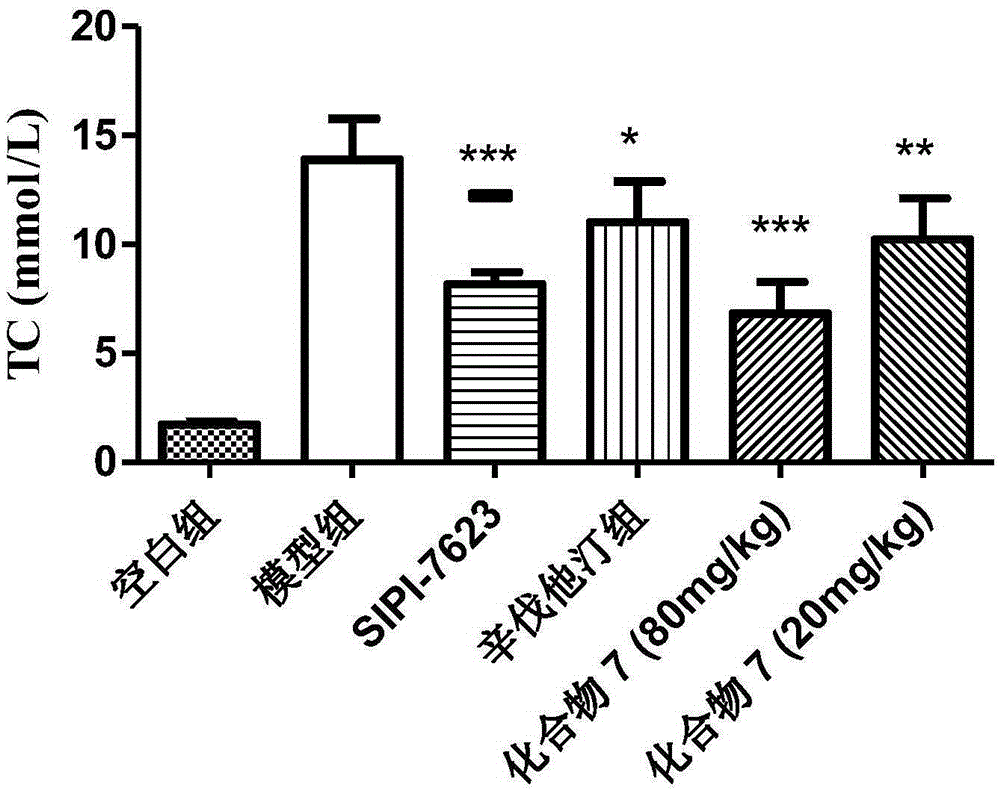
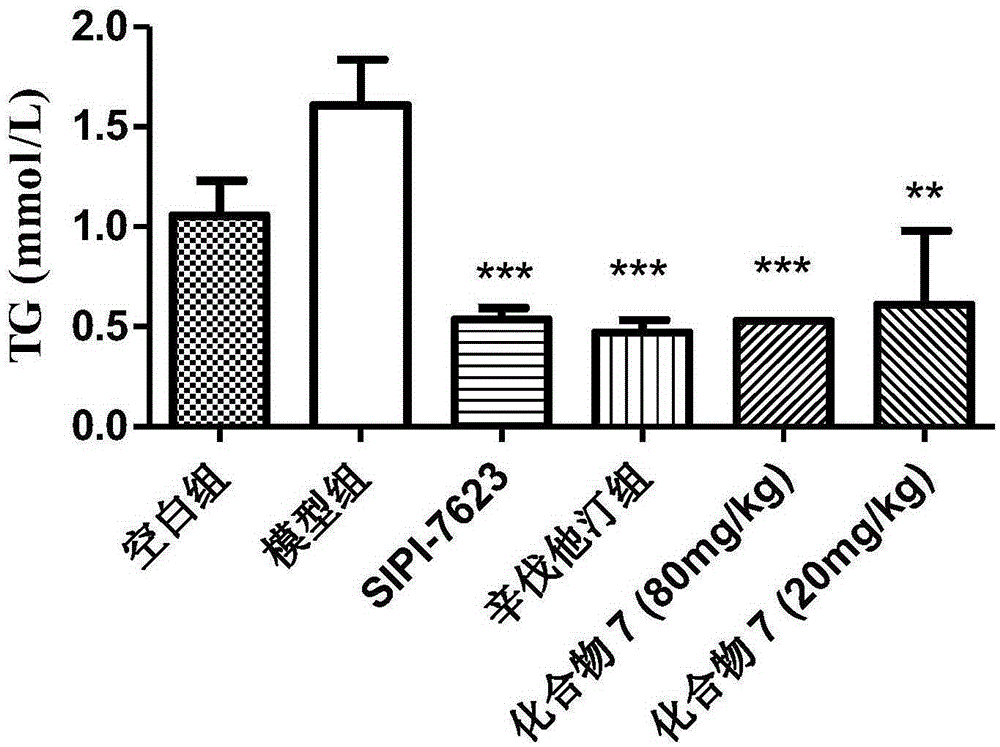
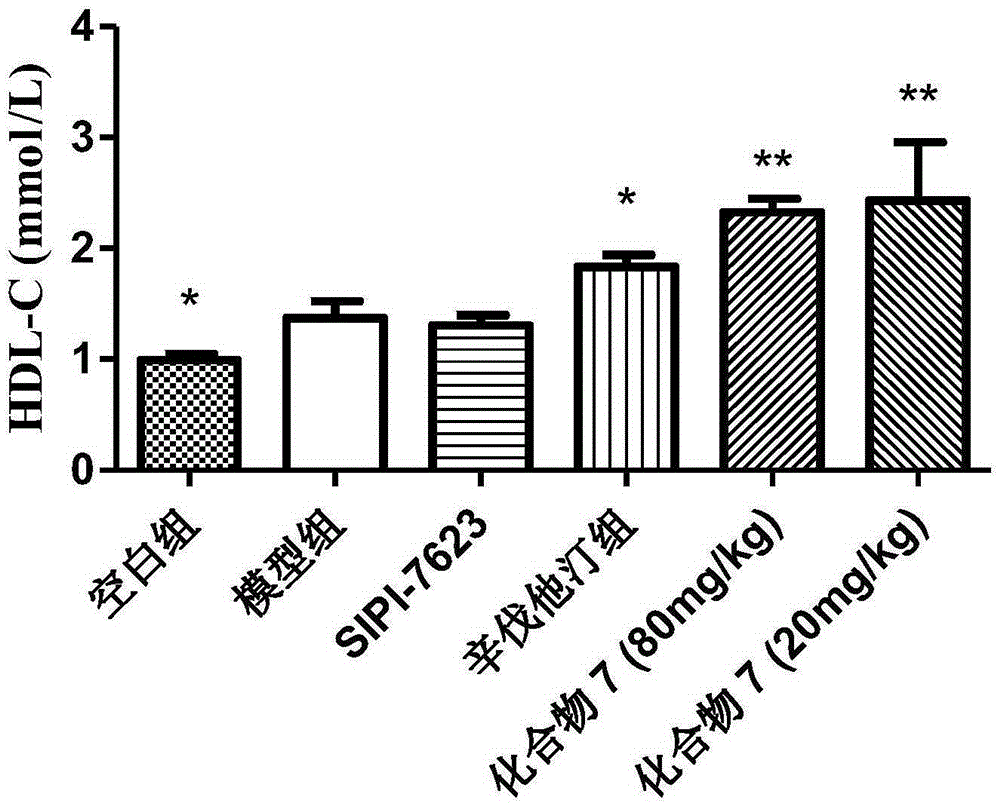
Carboxylic acid derivative and application of carboxylic acid derivative serving as FXR (farnesoid X receptor) antagonist
A solvent and drug technology, applied in the field of new drug design and synthesis, can solve the problem of not being able to improve the plasma lipid density of patients with high cholesterol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of compound 2-methyl-2-(4-(4-chlorobenzoyl)-phenoxy)-propionyl chloride shown in formula (III)
[0041]
[0042] Dissolve 31.8 g of 2-methyl-2-(4-(4-chlorobenzoyl)-phenoxy)-propionic acid in 200 mL of dichloromethane, add 1 mL of N,N-dimethylformamide and stir, Add 19.0 g of oxalyl chloride dropwise at 0°C, react at room temperature for 3 hours, spin out dichloromethane to obtain 2-methyl-2-(4-(4-chlorobenzoyl)-phenoxy)-propionyl chloride 30.8g, yield 91.4%.
Embodiment 2
[0043] Embodiment 2: the preparation of compound 1
[0044]
[0045] Dissolve 3.37g of 2-methyl-2-(4-(4-chlorobenzoyl)-phenoxy)-propionyl chloride in 25mL of 1,4-dioxane for later use, and 1.38g of p-hydroxybenzoic acid Add to 1,4-dioxane:water / 1:1 20mL mixed solvent, add 10mL 2N sodium hydroxide at 0°C, stir for 30 minutes, then add the reserved solution dropwise at 0°C, Stirring was continued for 10 hours, acidified, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and column chromatographed to obtain 3.51 g of the target compound with a yield of 80.1%. mp:183.1-185.2℃,ESI-MS m / z:437[M-H] + ,439[M+H] + . 1 H NMR (400MHz, DMSO) δ13.08(s, 1H), 8.01(d, J=8.4Hz, 2H), 7.80(d, J=8.4Hz, 2H), 7.74(d, J=8.4Hz, 2H ), 7.63(d, J=8.4Hz, 2H), 7.29(d, J=8.4Hz, 2H), 7.09(d, J=8.4Hz, 2H), 1.82(d, 6H).
Embodiment 3
[0046] Embodiment 3: the preparation of compound 2
[0047]
[0048] Dissolve 3.37g of 2-methyl-2-(4-(4-chlorobenzoyl)-phenoxy)-propionyl chloride in 25mL of 1,4-dioxane for later use, and 1.37g of p-aminobenzoic acid Add to 1,4-dioxane:water / 1:1 20mL mixed solvent, add 10mL 2N sodium hydroxide at 0°C, stir for 30 minutes, then add the reserved solution dropwise at 0°C, Stirring was continued for 10 hours, acidified, extracted with ethyl acetate, dried over anhydrous magnesium sulfate, and subjected to column chromatography to obtain 3.78 g of the target compound with a yield of 86.3%. ESI-MSm / z:436[M-H] + . 1 H NMR (400MHz, DMSO) δ10.33 (s, 1H), 7.87 (d, J = 8.4Hz, 2H), 7.79 (d, J = 8.4Hz, 2H), 7.74–7.68 (m, 4H), 7.59 (d,J=8.4Hz,2H),7.03(d,J=8.4Hz,2H),1.65(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com