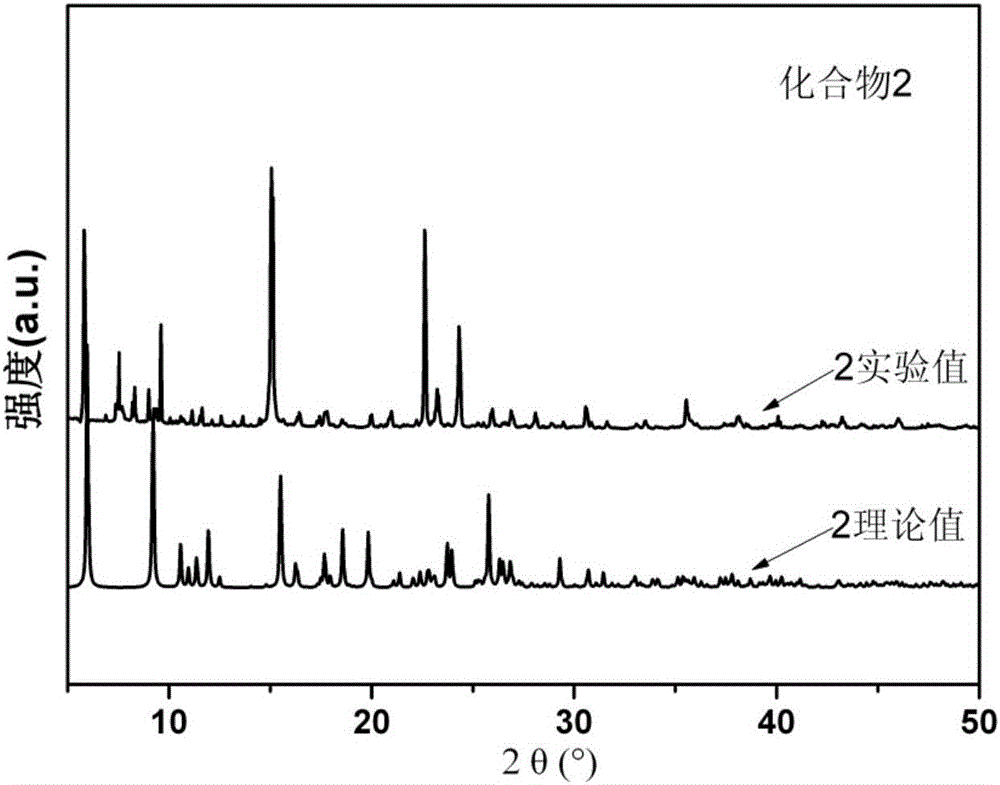
Multifunctional zinc/cadmium complex of thiophene-based organic ligand and application of multifunctional zinc/cadmium complex
A technology of organic ligands and cadmium complexes, applied in the direction of cadmium organic compounds, zinc organic compounds, 2/12 group organic compounds without C-metal bonds, etc., to increase rigidity and support capacity, increase sensitivity, reduce di The effect of secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Synthesis of [Zn(L)(1,3-bdc)] 2H 2 O·DMA, where L is N,N'-bis(3-pyridine)thiophene-2,5-dicarboxamide, the structural formula is: 1,3-bdc is isophthalate; DMA is N,N'-dimethylacetamide.
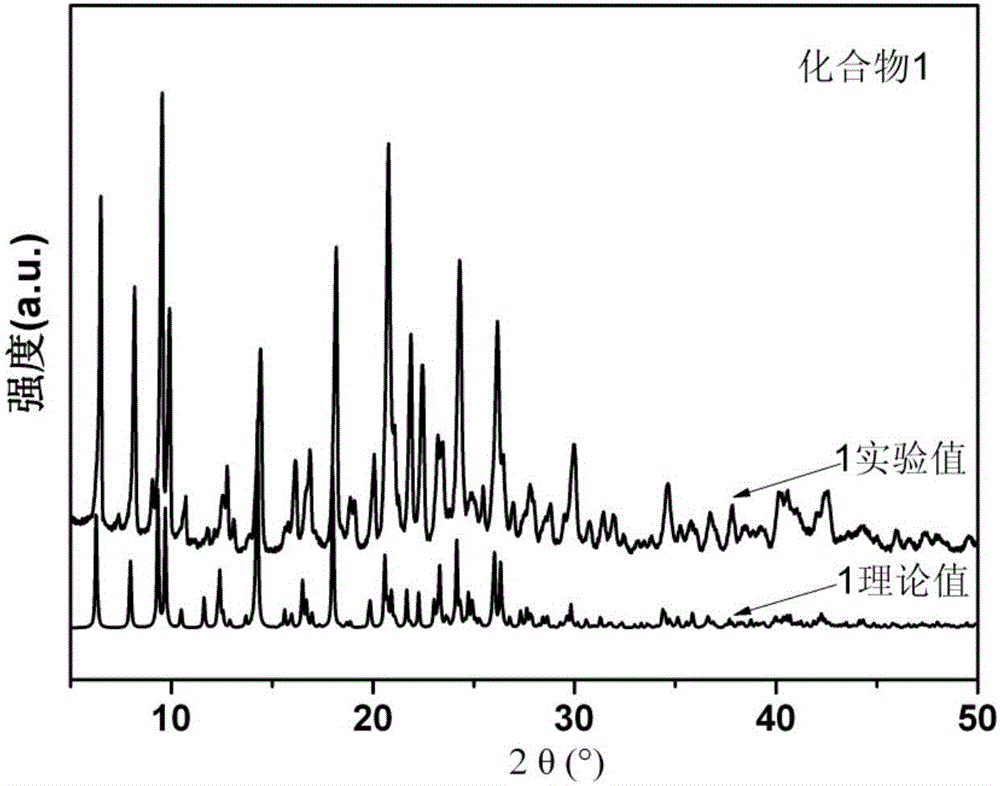
[0047] 0.1mmol Zn(NO 3 ) 2 ·6H 2 O, 0.10 mmol N,N'-bis(3-pyridine)thiophene-2,5-dicarboxamide, 0.1 mmol isophthalic acid, 5 mL DMA, and 1 mL H 2 O was sequentially added to a 25mL beaker, stirred at room temperature for 30min to obtain a clear solution, transferred to a 25mL autoclave, heated to 80°C at a heating rate of 1.25°C / h, kept at a constant temperature for 48h, and cooled at a rate of 1.25°C / h The temperature was lowered to room temperature to obtain colorless blocky crystals, washed twice with deionized water and ethanol alternately, and dried naturally at room temperature to obtain [Zn(L)(1,3-bdc)]·2H 2 O·DMA, the yield is 55%, and its XRD diffraction pattern is as figure 1 As shown, its coordination environment diagram is shown as Figure 6 As shown, its tw...
Embodiment 2
[0048] Example 2 Synthesis of [Zn(L)(1,3-bdc)] 2H 2 O·DMA, where L is N,N'-bis(3-pyridine)thiophene-2,5-dicarboxamide, 1,3-bdc is isophthalate, DMA is N,N'-di Methylacetamide.
[0049] 0.2mmol Zn(NO 3 ) 2 ·6H 2 O, 0.10 mmol N,N'-bis(3-pyridine)thiophene-2,5-dicarboxamide, 0.2 mmol isophthalic acid, 4 mL DMA, and 2 mL H 2 O was sequentially added to a 25mL beaker, stirred at room temperature for 45min to obtain a clear solution, transferred to a 25mL autoclave, heated to 85°C at a heating rate of 2.5°C / h, kept at a constant temperature for 54h, and cooled at a rate of 2.5°C / h The temperature was lowered to room temperature to obtain colorless blocky crystals, washed with deionized water and ethanol three times alternately, and dried naturally at room temperature to obtain [Zn(L)(1,3-bdc)]·2H 2 O·DMA, the yield is 85%, and its XRD diffraction pattern is as follows figure 1 As shown, its coordination environment diagram is shown as Figure 6 As shown, its two-dimensional s...
Embodiment 3
[0050] Example 3 Synthesis of [Zn(L)(1,3-bdc)]·2H 2 O·DMA, where L is N,N'-bis(3-pyridine)thiophene-2,5-dicarboxamide, 1,3-bdc is isophthalate, DMA is N,N'-di Methylacetamide.
[0051] 0.3mmol Zn(NO 3 ) 2 ·6H 2 O, 0.10 mmol N,N'-bis(3-pyridine)thiophene-2,5-dicarboxamide, 0.3 mmol isophthalic acid, 3 mL DMA, and 3 mL H 2 O was sequentially added to a 25mL beaker, stirred at room temperature for 60min to obtain a clear solution, transferred to a 25mL autoclave, heated to 90°C at a heating rate of 5°C / h, kept at a constant temperature for 96h, and cooled at a rate of 5°C / h The temperature was lowered to room temperature to obtain colorless blocky crystals, washed with deionized water and ethanol four times alternately, and dried naturally at room temperature to obtain [Zn(L)(1,3-bdc)]·2H 2 O·DMA, the yield is 65%, and its XRD diffraction pattern is as follows figure 1 As shown, its coordination environment diagram is shown as Figure 6 As shown, its two-dimensional struct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com