Calixarene chitosan polymer and preparation method thereof
A technology of calixarene and chitosan, which is applied in the field of calixarene-chitosan polymer and its preparation, can solve the problems of poor metal ion selectivity and poor adsorption effect, and achieve the goal of overcoming poor solubility and wide application Value, the effect of enhancing the metal adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
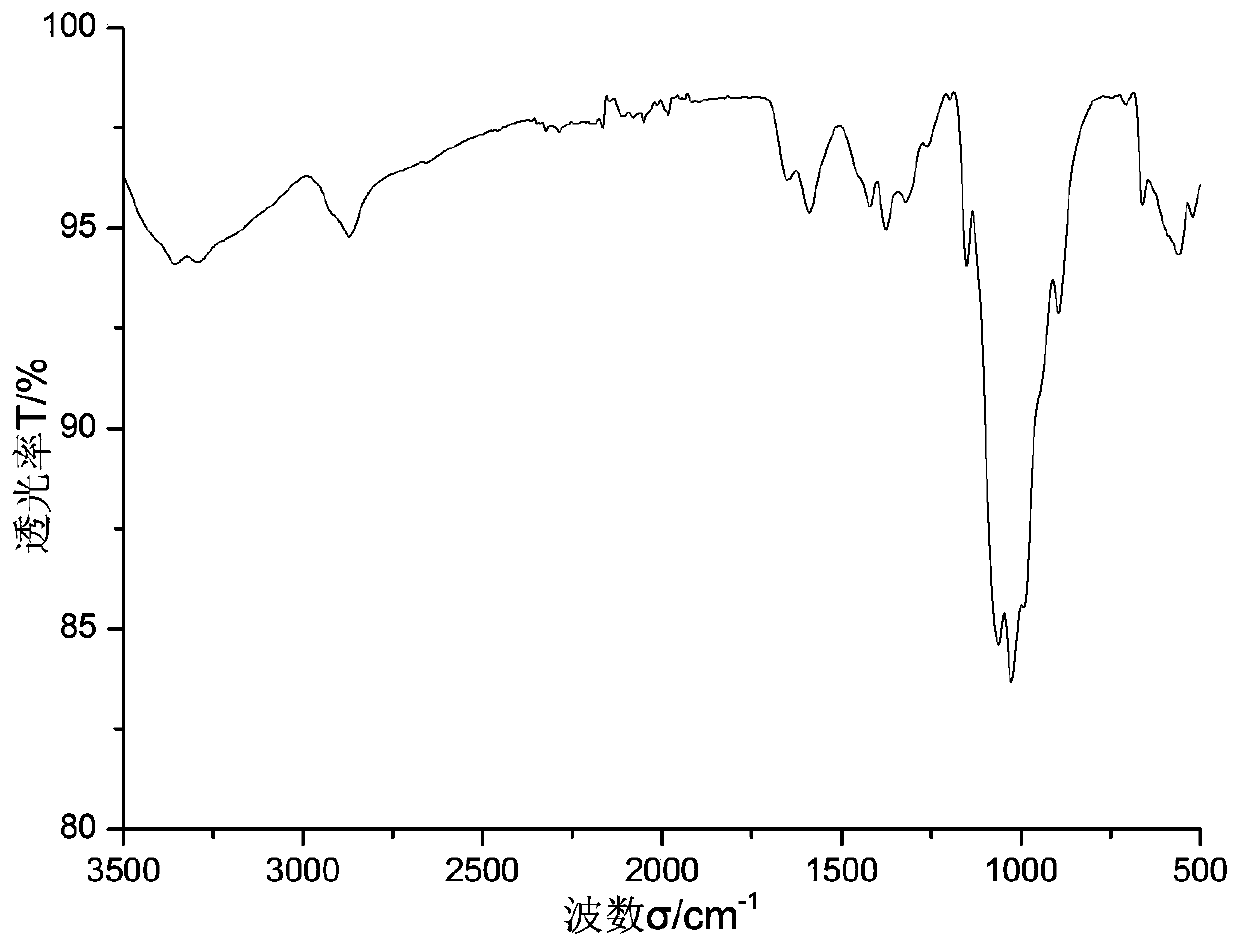
[0042] Add 0.6489 g of calix[4]arene and 0.12 mL of pyridine to 20 mL of dichloromethane solvent under nitrogen protection in an ice-water bath, stir with a magnet, and slowly drop in 0.175 mL of trifluoromethanesulfonic anhydride. After complete drop-in, react at room temperature for 2 hours, monitor by TLC to ensure that the raw material calix[4]arene completely disappears, the liquid is concentrated, dissolved in ether, and extracted with water several times to obtain calix[4]arene trifluoromethanesulfonate solid by filtration Dry and set aside. See formula 1 for the structural formula, wherein R is -OTf.
[0043] Add 0.39 g calix[4]arene triflate, 0.1612 g chitosan, 0.0056 g palladium acetate, 0.0234 g BINAP ligand, 0.2281 g cesium carbonate, in 10 mL of anhydrous THF and 15 mL of anhydrous DMSO In the solvent, react at a constant temperature of 90° C. for five hours under the protection of nitrogen. After cooling, part of the solvent was distilled off under reduced pres...
Embodiment 2
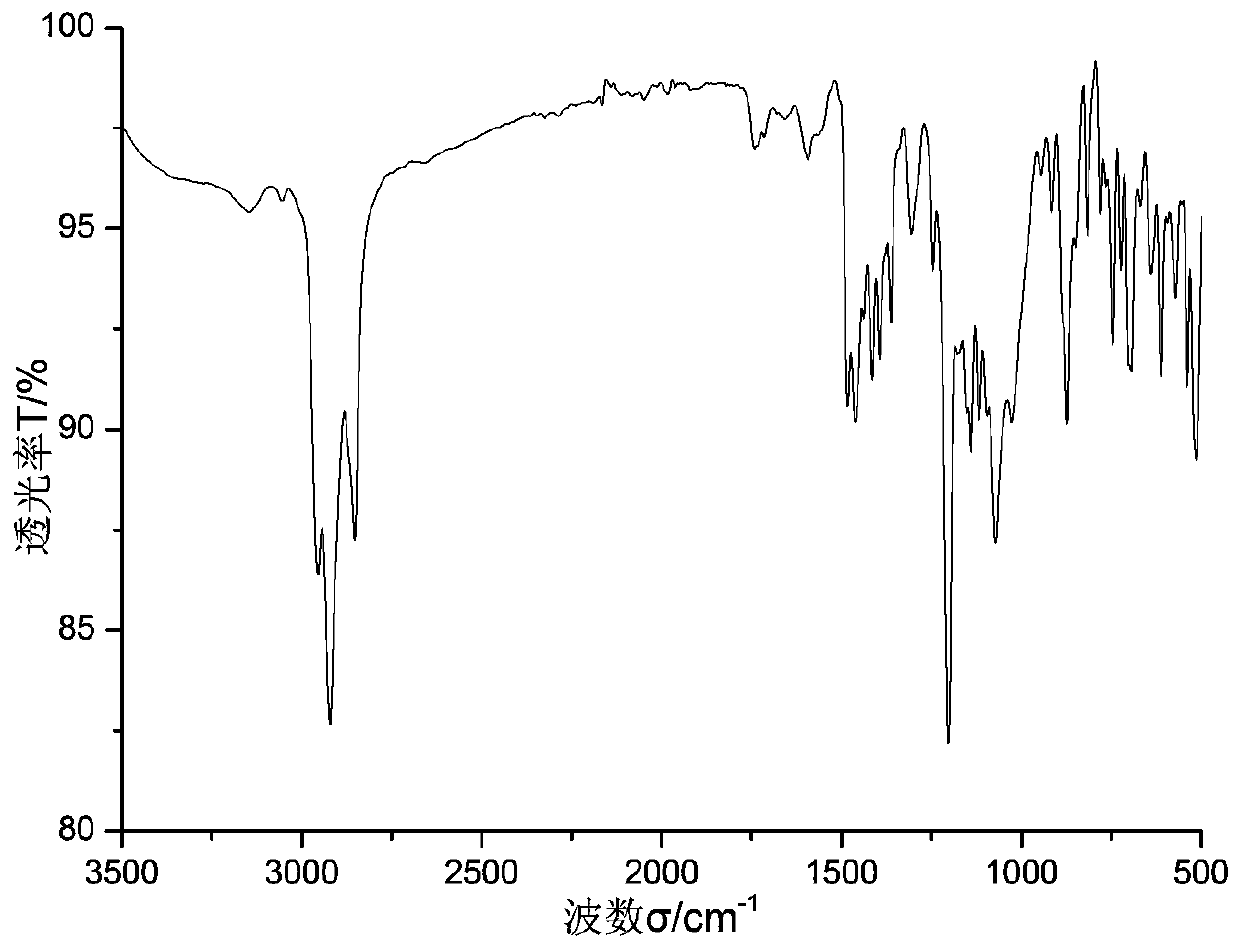
[0047] Add 0.9734 g of calix[6]arene and 0.12 mL of pyridine to 20 mL of dichloromethane solvent under nitrogen protection in an ice-water bath, stir with a magnet, and slowly drop in 0.175 mL of trifluoromethanesulfonic anhydride. After complete drop-in, react at room temperature for 2 hours, monitor by TLC to ensure that the raw material calix[6]arene completely disappears, the liquid is concentrated, dissolved in ether, and extracted with water several times to obtain calix[6]arene trifluoromethanesulfonate solid by filtration Dry and set aside. See formula 1 for the structural formula, wherein R is -OTf.
[0048] Add 0.5527 g calix[6]arene triflate, 0.1612 g chitosan, 0.0056 g palladium acetate, 0.0234 g BINAP ligand, 0.2281 g cesium carbonate in 10 mL of anhydrous THF and 15 mL of anhydrous dimethylsulfoxide In the solvent, react at a constant temperature of 90° C. for five hours under the protection of nitrogen. After cooling, part of the solvent was distilled off unde...
Embodiment 3
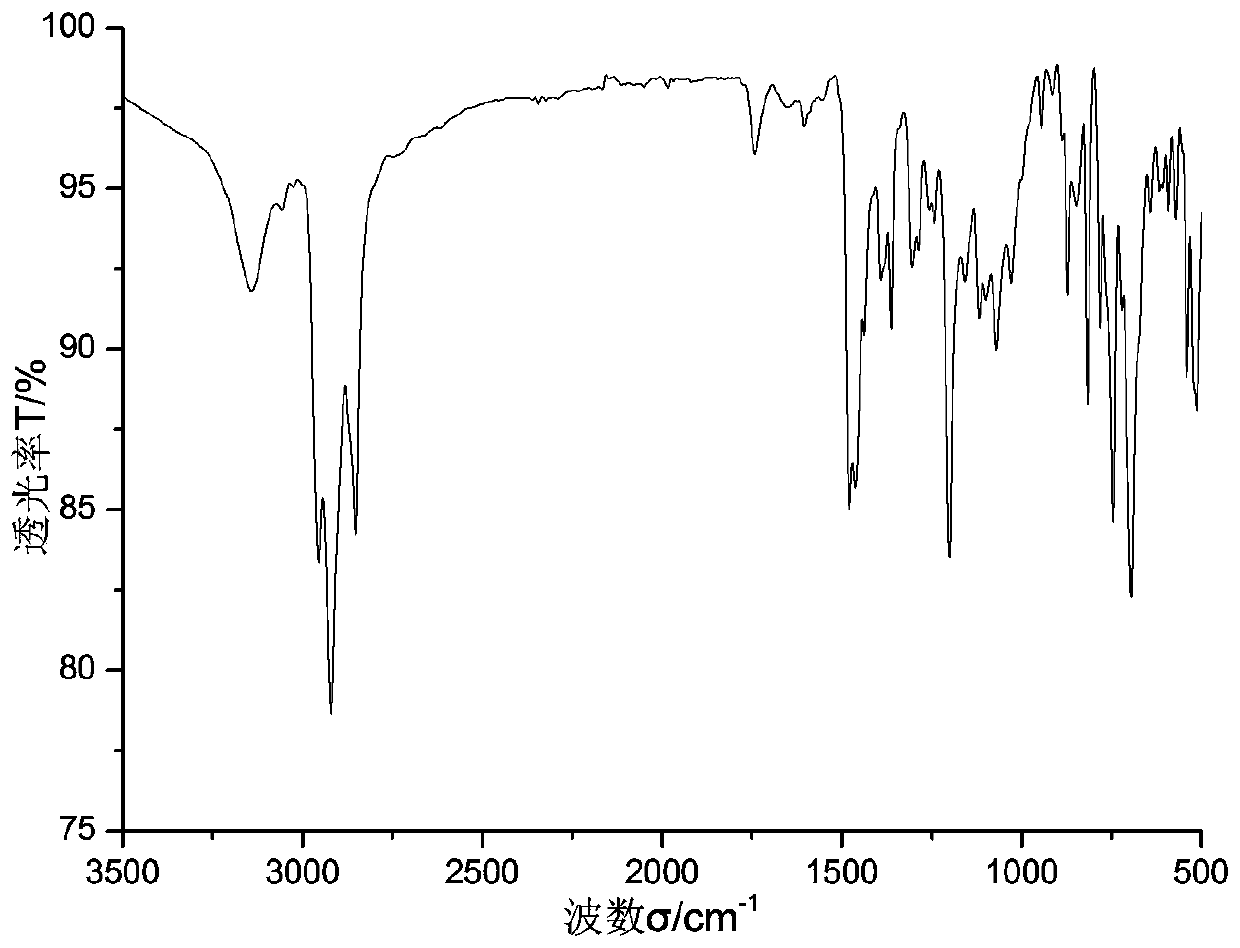
[0052] Add 1.2978 g of calix[8]arene and 0.12 mL of pyridine to 20 mL of dichloromethane solvent under nitrogen protection in an ice-water bath, stir with a magnet, and slowly drop in 0.175 mL of trifluoromethanesulfonic anhydride. After complete drop-in, react at room temperature for 2 h, monitor by TLC to ensure complete disappearance of the raw material calix[8]arene, concentrate the liquid, dissolve in ether, add water for extraction several times, and obtain calix[8]arene triflate as a solid by filtration Dry and set aside. See formula 1 for the structural formula, wherein R is -OTf.
[0053] Add 0.705 g calix[8]arene triflate, 0.1612 g chitosan, 0.0056 g palladium acetate, 0.0234 g BINAP ligand, 0.2281 g cesium carbonate in 10 mL of anhydrous THF and 15 mL of anhydrous dimethylsulfoxide In the solvent, react at a constant temperature of 90° C. for five hours under the protection of nitrogen. After cooling, part of the solvent was distilled off under reduced pressure, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com