Illuminating tetra-ligand trapezoid organic boron compound, and preparation method and application thereof
A compound and four-coordination technology, applied in the field of luminescent four-coordinate trapezoidal organoboron compound and its preparation, can solve the problems of unreported applications and few boron compounds, and achieve high carrier mobility and strong blue light emission , the effect of high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
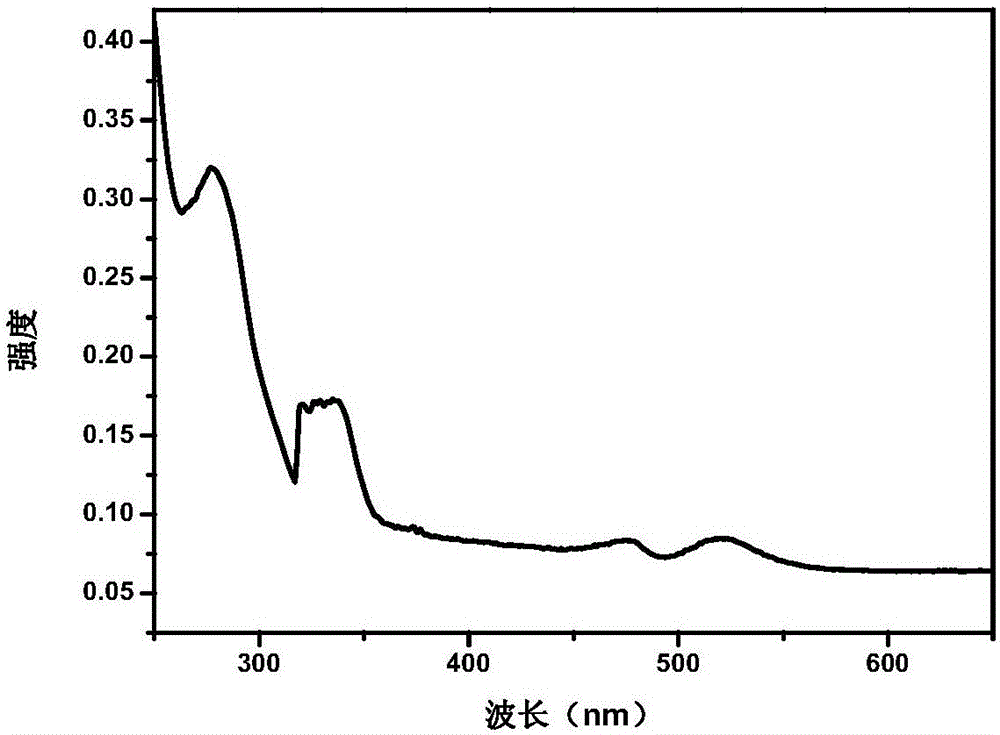
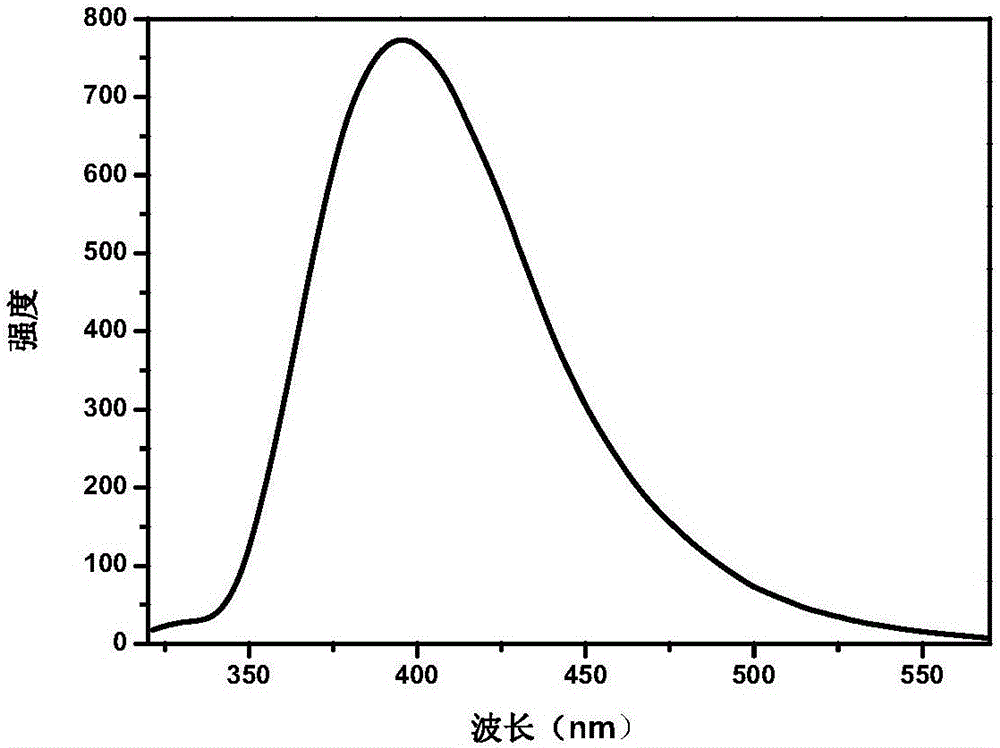
Image
Examples
Embodiment 1
[0043] When the structure of R is When, the preparation of compound 6a:
[0044] The synthetic route is as follows:
[0045]
[0046] Preparation of Alkynyl Compound 2:
[0047]
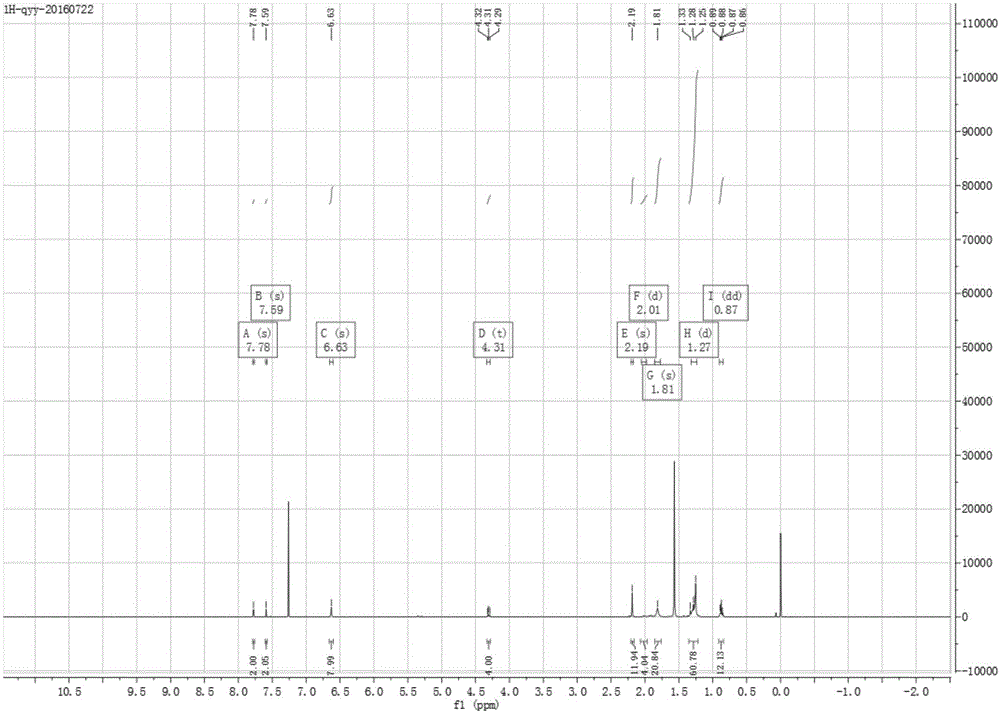
[0048] Take a dry reaction flask, add 1,4-dibromo-2,5-diiodobenzene (0.487g, 1mmol), trimethylethynyl silicon (0.589g, 6mmol), cuprous iodide (6mg, 0.03 mmol), tetrakistriphenylphosphopalladium (10mg, 0.1mmol), vacuumize on the double-row tube - keep nitrogen gas - vacuumize, cycle back and forth three times, and finally use nitrogen to protect the entire reaction system. Inject dry diisopropylamine (25 mL) redistilled in advance with a needle tube, and react in a closed manner at 90°C for 24 hours. Extract with water and ethyl acetate, take the organic layer, anhydrous NaSO 4 Dried, spin-dried, and passed through the column to obtain the corresponding alkynyl compound 2; 1 HNMR (400MHz, CDCl 3 )δ7.39(s,2H),0.08(s,18H),
[0049] Preparation of Compound 3 Containing Micylboron:
[0050]...
Embodiment 2
[0066] When the structure of R is When, the preparation of compound 6b:
[0067] The synthetic route is as follows:
[0068]
[0069] The preparation of the alkynyl compound 2, the phenynylsilicon compound 3 containing amygylboron and the compound 4 containing an alkyne in this example is the same as that in Example 1.
[0070] Preparation of compound 5b:
[0071]
[0072] NaN 3 (0.3575g, 5.5mmol) was dissolved in anhydrous DMSO (10mL), then benzyl bromide (0.855g, 5mmol) was added, and the reaction was stirred at 40°C for 5h. After the reaction was complete, the reaction mixture was transferred to a separatory funnel, and water (30 mL) and dichloromethane (30 mL) were added. The organic layer was washed with water (30 mL), anhydrous Na 2 SO 4 Dry and finally collect with a rotary evaporator. 1 HNMR (400MHz, CDCl 3 )δ7.36-7.33(m,4H),7.26(m,1H),2.67(m,2H).
[0073] Preparation of compound 6b:
[0074]
[0075] Weigh dimethyl borophenylacetylene (60mg, 0.17mm...
Embodiment 3
[0077] When the structure of R is When, the preparation of compound 6c:
[0078] The synthetic route is as follows:
[0079]
[0080] The preparation of the alkynyl compound 2, the phenynylsilicon compound 3 containing amygylboron and the compound 4 containing an alkyne in this example is the same as that in Example 1.
[0081] Preparation of compound 5c:
[0082]
[0083] NaN 3 (0.3575g, 5.5mmol) was dissolved in anhydrous DMSO (10mL), then bromonaphthalene (1.105g, 5mmol) was added, and the reaction was stirred at 40°C for 5h. After the reaction was complete, the reaction mixture was transferred to a separatory funnel, and water (30 mL) and dichloromethane (30 mL) were added. The organic layer was washed with water (30 mL), anhydrous Na 2 SO 4 Dry and finally collect with a rotary evaporator.
[0084] Preparation of compound 6c:
[0085]
[0086] Weigh dimethyl borophenylacetylene (60mg, 0.17mmol) and cuprous iodide (32.4mg, 0.17mmol) into a dry 100mL singl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com