Lactobacillus reuteri and applications
A technology of Lactobacillus reuteri and antibacterial drugs, applied in the field of microorganisms, can solve problems such as poor effect of enteritis, and achieve the effects of good development and application prospects, easy operation, and strong resistance to stress.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The isolation of embodiment 1 bacterial strain
[0038] 1. Strains, medium and culture conditions
[0039] (1) Bacterial source
[0040] Feces of healthy soldiers from a military region in China.
[0041] (2) culture medium
[0042] Plate medium: 1000mL distilled water, 10g peptone, 20g glucose, 9g yeast powder, 5g sodium acetate, 2g dipotassium hydrogen phosphate, 2g triammonium citrate, 0.5g cysteine, 0.2g magnesium sulfate, 0.05g manganese sulfate, Tween 1ml, agar powder 15-20g, pH5.5-6.5.
[0043] 2. Isolation and identification of strains
[0044] (1) Isolation of strains
[0045] Take an appropriate amount of fresh feces from the pre-sterilized glove box and place them in a sterile test tube filled with sterile medium, vortex and mix well;
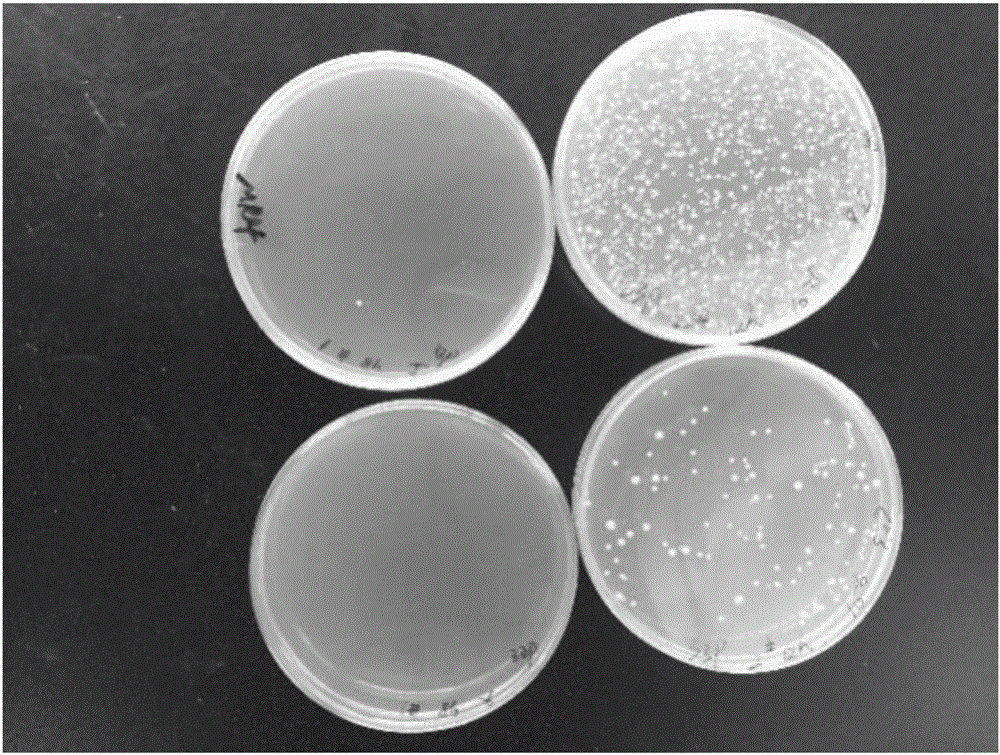
[0046] Take the feces solution that was fully shaken and vortexed as the stock solution, and make a ten-fold gradient dilution to a suitable gradient, take 100 μl and evenly spread it on the MRS+0.05% cysteine solid pl...
Embodiment 2
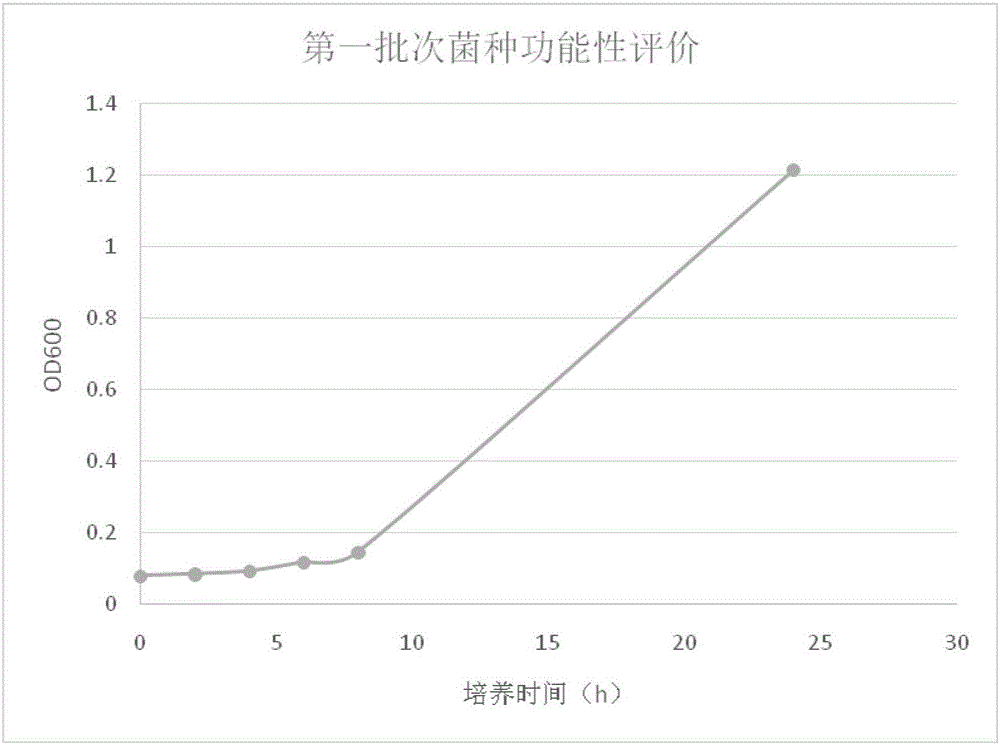
[0066] (1) Functional evaluation of strains
[0067] A. Determination of growth curve and pH value of each monitoring point
[0068] 1. mark
[0069] Take 11 large sterile test tubes and mark the incubation time with a marker pen, namely 0, 2, 4, 6, 8, and 24 hours.
[0070] 2. Vaccination
[0071] Use 5mL sterile pipettes to draw 2.5mL TR02 overnight culture solution (cultured for 8-16h) into the Erlenmeyer flask filled with 50mL MRS+0.05% cysteine culture solution, mix well and take 5mL of the mixed solution into the above-mentioned Labeled 11 large sterile test tubes.
[0072] 3. Cultivate
[0073] Place the inoculated test tubes in a 37°C incubator for static culture, and culture them for 0, 2, 4, 6, 8, and 24 hours respectively. Take out the test tubes marked with the corresponding time and store them in the refrigerator immediately. optical density value.
[0074] 4. Nephelometric assay
[0075] Use uninoculated MRS+0.05% cysteine medium as blank control, choo...
Embodiment 3
[0104] Embodiment 3 in vivo experiments
[0105] 1. Induction and treatment of colitis
[0106] C57BL / 6 type mice (1-7 days) were fed with a solution containing 2.5% DSS (molecular weight 36-50KDa) to induce colitis model mice; the control group was fed water. In the following 10 days, water, lactic acid bacteria (embedded 0.9*10 9 , 1.2*10 9 , 2.4*10 9 , unembedded 2.4*10 9 ) and sulfasalazine pyridine (0.5g / kg).
[0107] Weigh the body weight every day, observe the consistency of the feces and whether there is blood in the feces and the anus of the mice. Disease activity index (DAI) was calculated. In short, calculations are performed with the following parameters:
[0108] a) Diarrhea (0 = normal, 2 = loose stools, 4 = watery diarrhea);
[0109] b) Blood gallbladder (0 points = no bleeding, 2, slight bleeding, 4 points, heavy bleeding).
[0110] On day 10 after induction of colitis, animals were sacrificed, colons were removed, and colon tissue pieces were prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com