Recombinant retroviral vector and application thereof
A technology of retroviruses and vectors, applied in the field of genetic engineering, can solve problems that need to be further improved and the improvement range is not large, and achieve the effects of improved effects, stable expression levels, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Construction of retroviral vector of the present invention
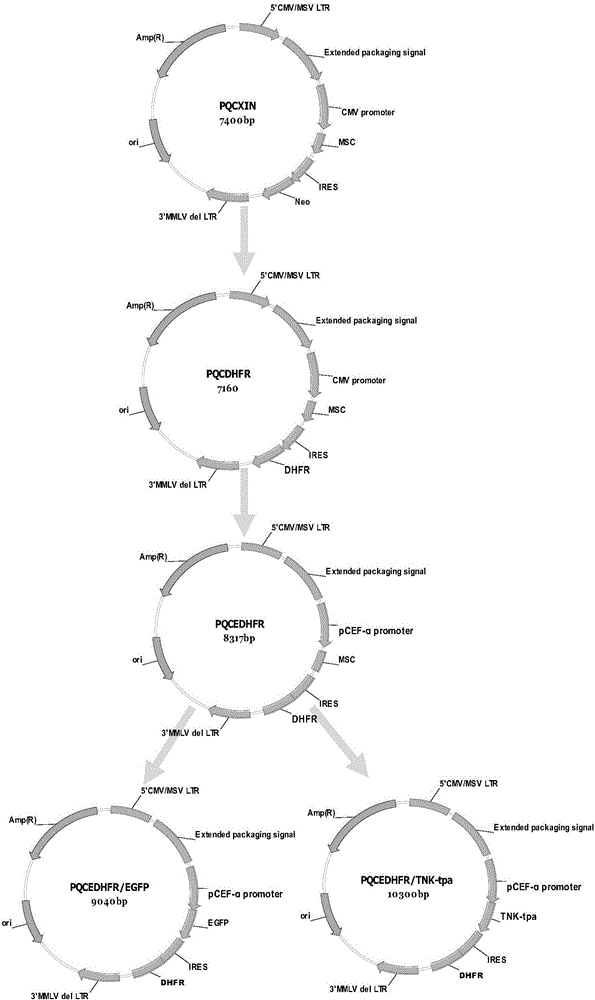
[0029] Such as figure 1 Shown to construct the retroviral vector PQCEDHFR of the present invention:
[0030] Take the retroviral vector PQCXIN (its structure is as figure 1 As shown, purchased from clontech company), using restriction sites AgeI (available from NEB company) and XhoI (available from NEB company) to excise IRES and Neo;
[0031] Connect the two genes of IRES and DHFR (sequence shown in SEQ NO:1) by overlap extension PCR method to obtain the gene fragment IRESDHFR. Design restriction sites AgeI and XhoI at both ends, and use restriction sites AgeI for PQCXIN Link with XhoI to remove IRES and Neo to construct the vector PQCDHFR;
[0032] Using the XbaI (purchased from NEB company) and NotI (purchased from NEB company) restriction sites on the vector PQCDHFR, the promoter CMV was replaced with the PCEF promoter (sequence shown in SEQ NO: 2) to construct the reverse transcription of the present in...
Embodiment 2
[0034] Example 2 Transfection of cells with retroviral vector of the present invention carrying EGFP gene
[0035] 1. Experimental materials
[0036] The retroviral vector PQCEDHFR of the present invention (the nucleotide sequence is shown in SEQ NO: 3): prepared according to the method of Example 1.
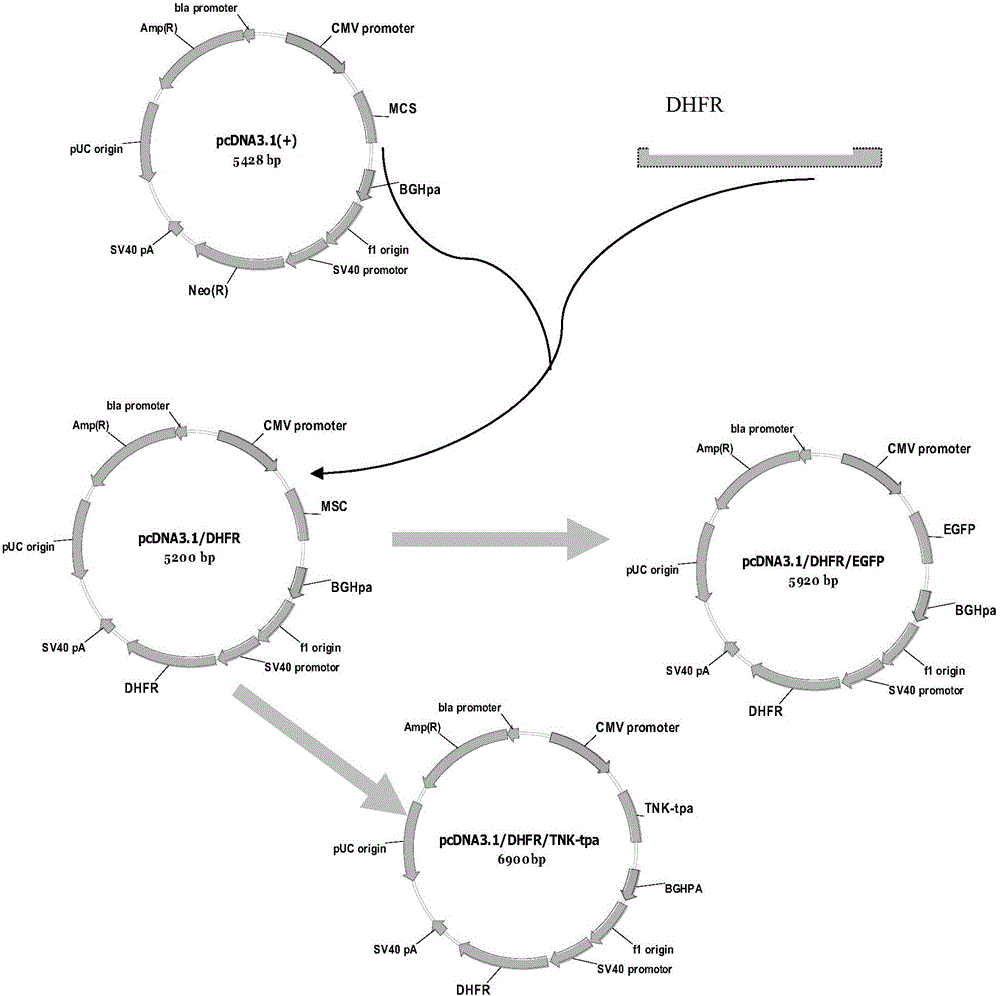
[0037] Control plasmid: pcDNA3.1(+) (purchased from Invitrogen) was digested with XmaI (purchased from NEB) and BstBI (purchased from NEB) by conventional molecular biology methods to remove the screening gene Neo, and then connected to The DHFR gene was cut with the same restriction enzymes, and the vector pcDNA3.1 / DHFR was successfully constructed, see attached figure 1 And SEQ NO: 4.
[0038] 2. Experimental method
[0039] Connect the EGFP reporter gene at EcoRV of pcDNA3.1 / DHFR and name it pcDNA3.1 / DHFR / EGFP as attached figure 2 As a control vector, the vector PQCEDHFR was digested with NotI (purchased from NEB) and Pad (purchased from NEB), and then connected to the same digested ...
Embodiment 3
[0055] Example 3 Transfection of cells with the retroviral vector of the present invention carrying TNK-tpa gene
[0056] 1. Experimental materials
[0057] In order to further verify the feasibility of this method, the foreign gene TNK-tpa was used as the test gene construction vector pcDNA3.1 / DHFR / TNK-tpa and PQCEDHFR / TNK-tpa( figure 1 , 2 ).
[0058] 2. Experimental method
[0059] According to the transfection and infection methods and the method of screening pressurized amplification genes in Example 2, a mixed clone population of positive cells expressing the TNK-tpa gene was obtained.
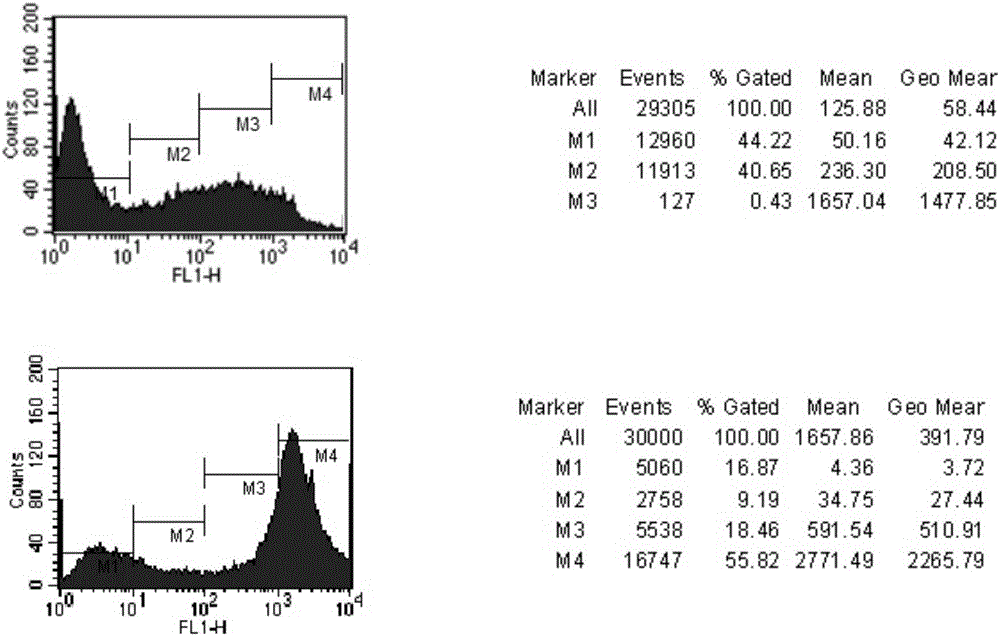
[0060] Separately monoclonal cells into a 96-well plate by limiting dilution. When the monoclonal cells grow to 1 / 2-2 / 3 of the entire well plate in about 2 weeks, use the in vitro fibrin agar plate lysis method to detect TNK-tPA In vitro fibrinolytic activity, the expression of cell supernatant was determined, and 10 high-expressing cell lines were selected respectively. The results are shown in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com