Engineering cell strains capable of stably and efficiently expressing HCV NS3 protein antibody and application thereof
A high-efficiency expression, cell line technology, applied in genetically modified cells, cells modified by the introduction of foreign genetic material, applications, etc., to achieve the effect of stable expression level, high sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Acquisition of engineered cell lines stably and efficiently expressing HCV NS3 protein antibody
[0037] A pair of monoclonal antibodies 3A10B5 and 7E3B6 secreted by two hybridoma cells CCTCC NO.C2018227 and CCTCC NO.C2018228 obtained through experimental screening entrusted a sequencing company (Shanghai Boshang Biotechnology Co., Ltd.) to perform nucleotide sequencing of the antibodies.
[0038] Determine the nucleotide sequence of the light chain of the monoclonal antibody 3A10B5 produced in CCTCC NO.C2018227 hybridoma cell line as shown in SEQ ID NO.5, and the nucleotide sequence of its heavy chain as shown in SEQ ID NO.6, while It is also assumed to be a nucleic acid molecule encoding the genetically engineered antibody NS3-GC1, the nucleotide sequence of its light chain is also shown in SEQ ID NO.5, and the nucleotide sequence of its heavy chain is also shown in SEQ ID NO.6.
[0039] Determine the nucleotide sequence of the light chain of the monoclonal...
Embodiment 2
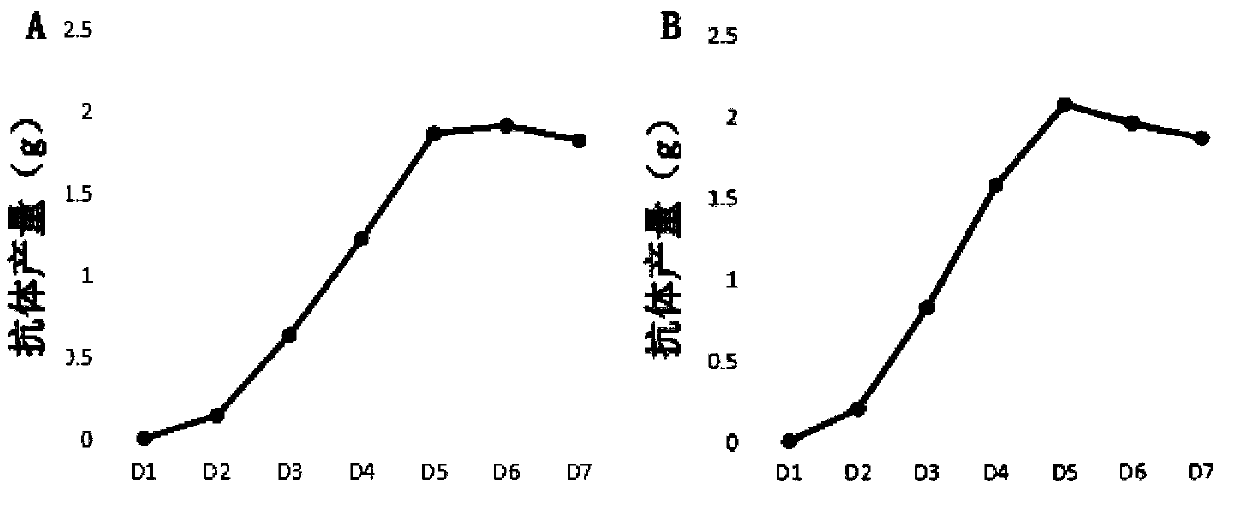
[0077] Preparation and purification of embodiment 2 genetic engineering antibody
[0078] 2.1 Batch culture of cell lines CHO / NS3-GC1 and CHO / NS3-GC2
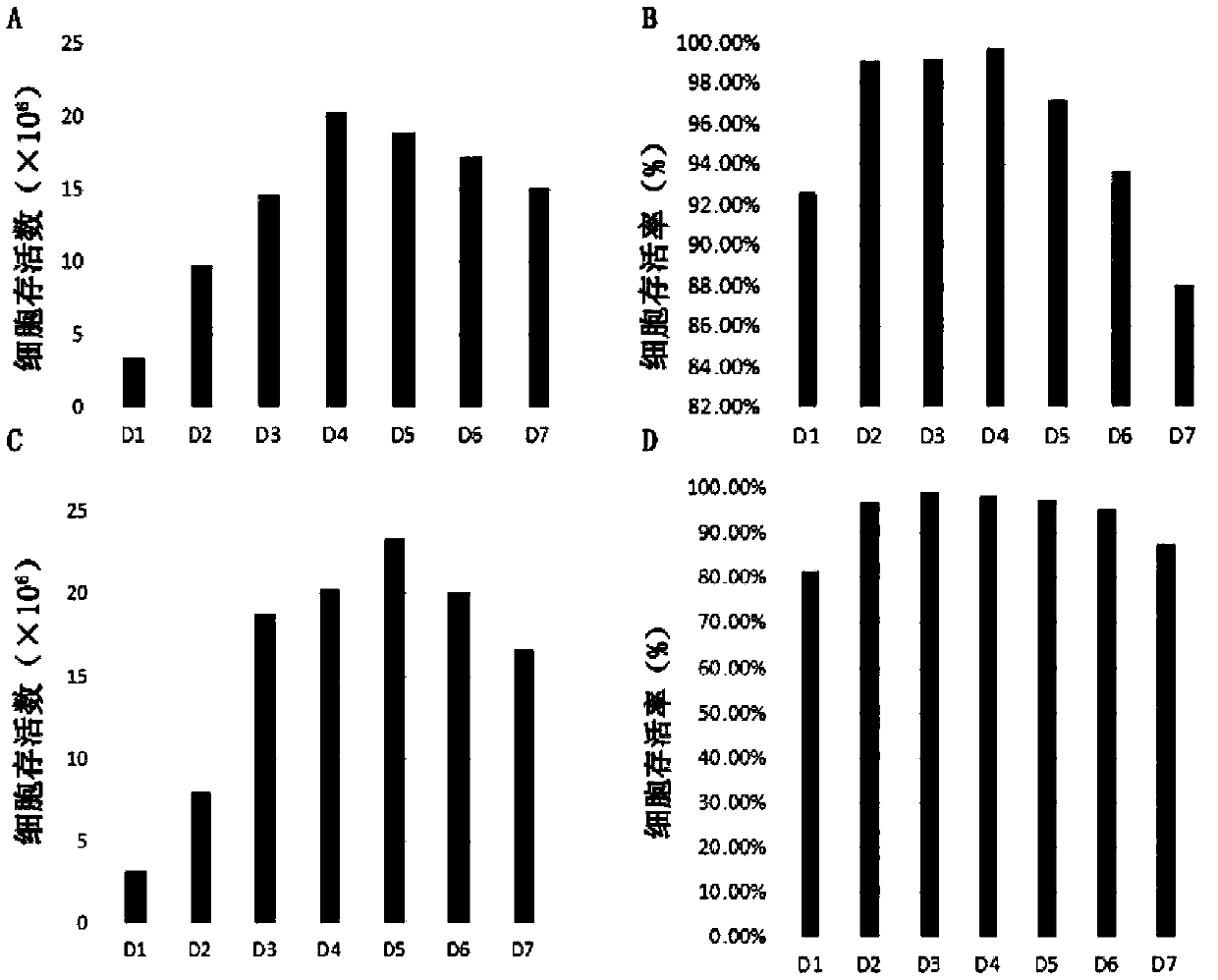
[0079] The cell lines CHO / NS3-GC2 and CHO / NS3-GC2 were divided into 0.4×10 6 The density of cell / mL was inoculated into the SF250ml cell culture Erlenmeyer flask, the culture system was 50mL, and the medium used was a special medium (CHO-Med-01) that could significantly increase the antibody production, and its basic formula was: CHO-S-SFM II Dry powder medium 10g / L, NaHCO 3 2.45g / L, 8mM glutamine, 2mM ferric citrate, final pH 7.1. After inoculation, hope blue staining and counting were carried out, and the culture bottle was placed in a cell shaker incubator at 37° C., relative humidity 70-80%, and 5% carbon dioxide, and the rotation speed was 125 rpm.
[0080] Observe the cell growth every day, trypan blue staining and counting, first take 20 μL, add 20 μL of 0.4% trypan blue staining solution to a centrifuge tube and mix...
Embodiment 3
[0089] Example 3 Antibody Identification
[0090] 3.1 Antibody titer identification
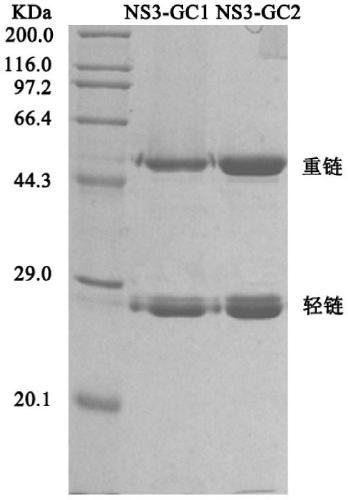
[0091] Weigh 5 mg of HRP and dissolve in 1 mL of distilled water; add 0.5 mL of freshly prepared 0.06M NaIO to the above solution 4 Solution, let stand at 4°C for 30min; add 0.5mL of 0.16M polyethylene glycol to the above solution, and let stand at room temperature for 30min; add 5mg of purified antibody (NS3-GC1 or 3A10B5; NS3-GC2 or 7E3B6), mix well and transfer into a dialysis bag, and dialyze against 0.05M pH9.5 carbonate buffer overnight; add 0.2ml of 5mg / mL NaBH the next day 4 solution, after mixing, let stand at 4°C for 2 hours; slowly add an equal volume of saturated (NH4) 2 SO 4 Solution, stand at 4°C for 30 minutes, then centrifuge to remove the supernatant, dissolve the precipitate with a little 0.02M pH7.4 PBS solution, and transfer it to a dialysis bag, dialyze with the same liquid at 4°C overnight to desalt; take it out and centrifuge the next day, and take the supernatant ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com