Kit for left colonic carcinoma detection
A kit and technology for colon cancer, applied in biological testing, measuring devices, material inspection products, etc., to achieve the effects of low cost, easy preparation, and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
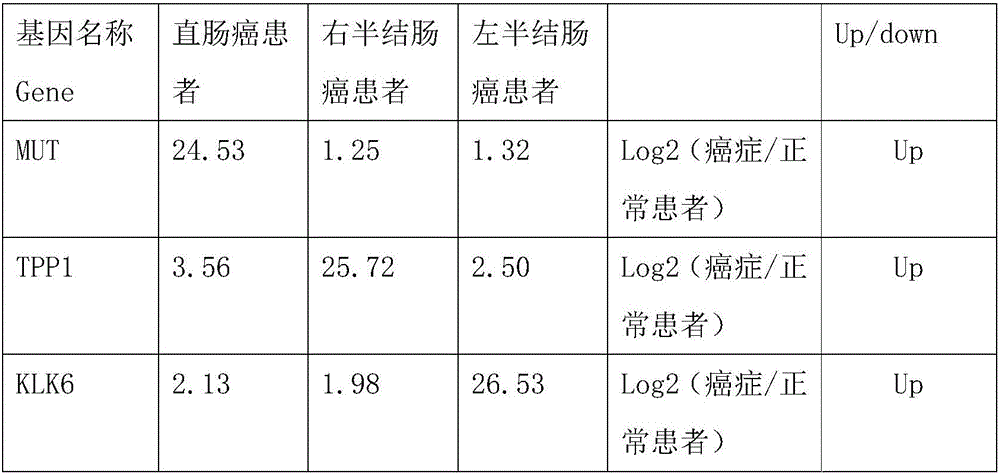
[0013] Example 1, screening and identification of rectal cancer markers
[0014] 1. Sample collection
[0015] 5mL peripheral blood samples were collected from 15 patients with rectal cancer clinically identified, 13 patients with right colon cancer and 10 patients with left colon cancer, anticoagulated and stored at -80°C. The patients mentioned above were all from the Cancer Hospital of Tianjin Medical University, and signed informed consent with the patients, which also met the ethical requirements.
[0016] 2. RNA extraction
[0017] Total RNA was extracted from colorectal cancer tissues and normal control tissues according to the operation instructions of the Trizol Reagent kit, dissolved in DEPC-treated water, and the concentration and purity of total RNA were measured by Nanodrop-1000 nucleic acid and protein analyzer, and stored at -80°C. Through detection, the RNA concentration is generally between 100ug-250ug / mL, which can be used for subsequent library establishme...
Embodiment 2
[0025] Screening and preparation of embodiment 2 KLK6 protein nucleic acid aptamer
[0026] Design a random nucleic acid library comprising approximately 20 nucleotides at both ends and 36 nucleotides in the middle as follows:
[0027] 5'-TCAAGCATGAATTGCAGAATGAC(N36)AGCAAGTGAGTAGCCAGTAAGCA-3'; N36 represents 36 random nucleotides.
[0028] (1) Firstly, the original random oligonucleotide library is amplified to increase its copy number to facilitate screening. The original random oligonucleotide library was amplified by PCR with upstream primers and biotin-modified downstream primers, using a PCR kit purchased from Shanghai Sangong Company. The reaction system is as follows:
[0029] Upstream primer 100pmol (5'-TCAAGCATGAATTGCAGAATGAC-3')
[0030] Downstream primer 100pmol (5,-biotin-TGCTTACTGGCTACTCACTTGCT-3,)
[0031] Template DNA 10μg (5'-TCAAGCATGAATTGCAGAATGAC(N36)AGCAAGTGAGTAGCCAGTAAGCA-3')
[0032] Wherein, the downstream primer is a biotin-modified downstream prim...
Embodiment 3
[0038] Example 3 The performance measurement of protein binding suitable gametes
[0039] Based on the property of graphene oxide oxidation to adsorb single-stranded DNA, a method for verifying the affinity of oligonucleotide aptamers was constructed. A fixed concentration of the KLK6 protein target (1 μM) was incubated with a series of corresponding candidate oligonucleotide aptamers of different concentrations (10, 25, 50, 75, 100, 150, 200 uM) in a total volume of 300 uL, Incubate at 37°C in the dark for 2 hours, and use BB buffer instead of the target as a negative control group. After incubation and binding, GO with the optimal dosage ratio was added to adsorb the aptamer that was not bound to the target. After centrifugation, the fluorescence intensity emitted by the supernatant at 520nm under 490nm excitation was measured with an F-7000 fluorescence photometer. Use dark treatment. Taking the fluorescence intensity of the experimental group relative to the negative con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com