Preparation method of negative electrode material of lithium-ion battery loaded with nano-boron on graphene
A technology for lithium-ion batteries and negative electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve problems such as inability to meet the needs of high-capacity electrodes, achieve good thermal and chemical stability, good conductivity, and negative electrode potential flat effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
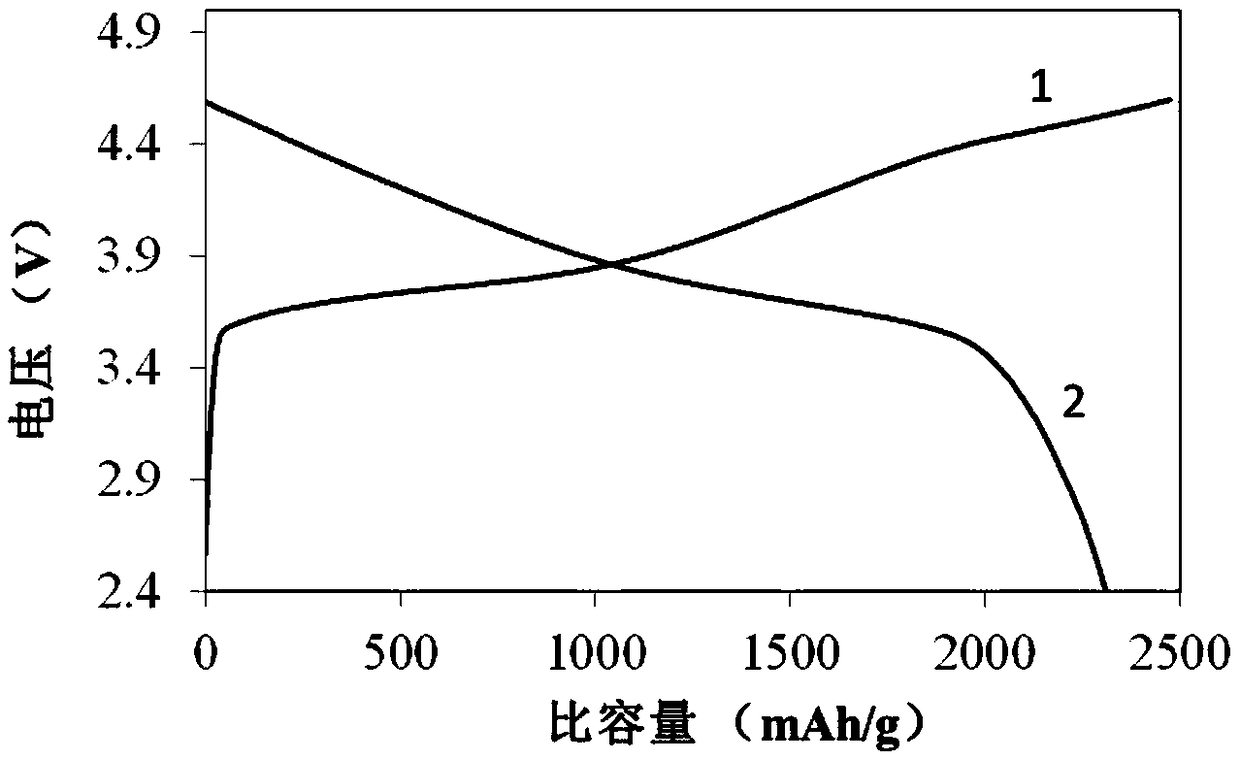
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of NaCl-KCl eutectic salt
[0032] Add NaCl (45g) and KCl (55g) into the ball mill jar, ball mill and mix at 700rpm for 1 hour, take it out and put it into a crucible, heat it in air at 700°C for 2 hours, and cool it to 25°C to obtain NaCl-KCl eutectic salt.
Embodiment 2
[0033] Example 2: Preparation of graphene-supported nano-boron precursor
[0034] Take the NaCl-KCl eutectic salt (10g) prepared in Example 1, add glucose monohydrate (0.4g), urea (0.2g), monohydrate monohydrate and Boric acid (0.1g) and NaCl-KCl eutectic salt (10g) were mixed by ball milling at a speed of 700rpm for 2 hours to prepare a precursor of graphene-loaded nano-boron.
Embodiment 3
[0035] Example 3: Preparation of Salt-Containing Graphene Loaded Nano Boron
[0036] Take the NaCl-KCl eutectic salt (20g) prepared in Example 1, add glucose monohydrate (0.4g), urea (0.2g), monohydrate monohydrate into the ball mill tank at a mass ratio of 4:2:1:200 Boric acid (0.1g) and NaCl-KCl eutectic salt (20g), rotating speed 700rpm ball milling and mixing for 2 hours, prepared graphene-loaded precursor of nano-boron;
[0037]The obtained precursor was heated to 110°C for 2 hours in a nitrogen atmosphere, then heated to 440°C for 3 hours, then heated to 1050°C, reduced for 2 hours and then cooled to 25°C to obtain salt-containing graphene-supported nano-boron. The heating rate for three times is 10°C / min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com