A fusion protein vaccine that inhibits streptococcal and/or prevents streptococcal infection
A fusion protein and protein technology, applied in the direction of bacteria, fungi, hybrid peptides, etc., can solve problems that hinder the development of type A streptococcal vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
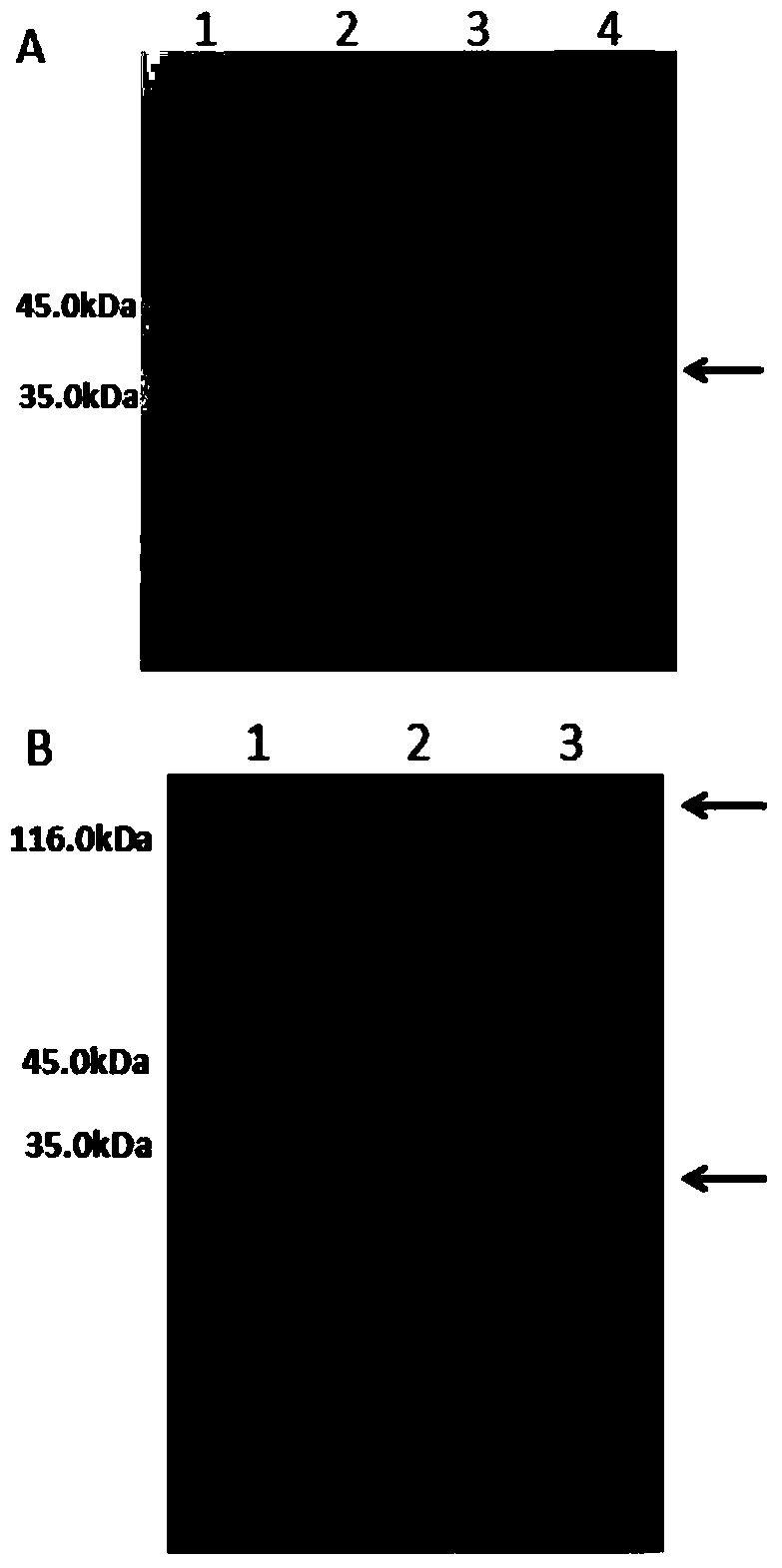
[0114] Embodiment 1, the construction of CTB-Sortase A protein gene and the preparation of CTB-Sortase A protein thereof
[0115]1. Replace the DNA fragment between the NcoI and BamHI recognition sequences of the vector pET26b (+) with the DNA sequence shown in the 112-996th position of SEQ ID No.2, keep other DNA sequences unchanged, and obtain the recombinant expression vector pET26b- CTB-Sortase A. Sequencing results show that the recombinant expression vector pET26b-CTB-Sortase A is inserted into the DNA sequence shown in the 112th-996th position of SEQ ID No.2, and the recombinant expression vector pET26b-CTB-Sortase A can express the first sequence of SEQ ID No.1. The CTB-Sortase A protein shown in the 23-314 position, the DNA sequence shown in the 115-996 position of SEQ ID No.2 is the encoding of the CTB-Sortase A protein shown in the 23-314 position of SEQ ID No.1 sequence.
[0116] 2. Transform the recombinant vector pET26b-CTB-Sortase A into BL21(DE3)plysS compete...
Embodiment 2
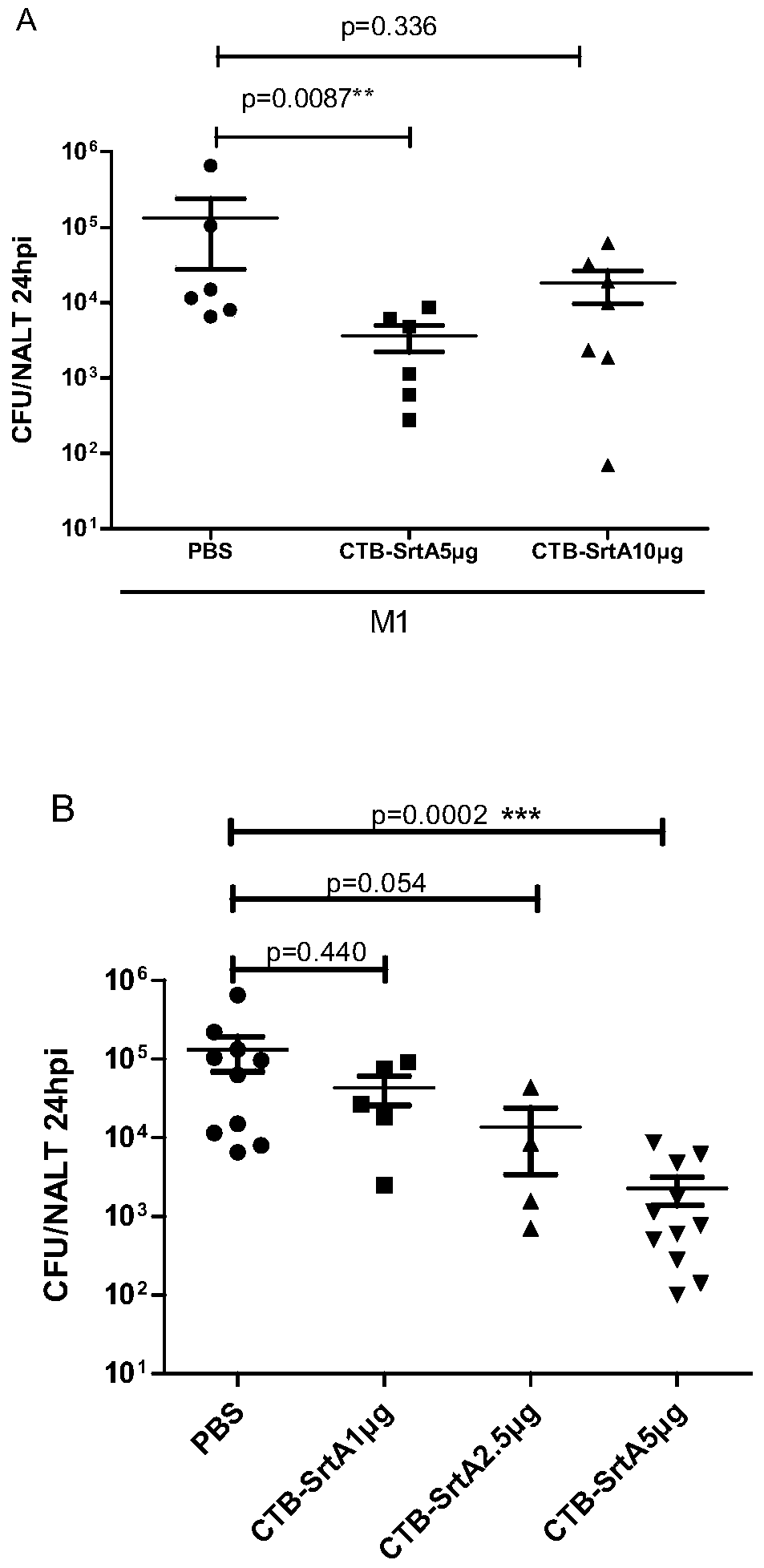
[0124] Example 2. Immunoprotection against Type A Streptococcus infection after nasal cavity inhalation of CTB-Sortase A protein
[0125] Female BALB / c mice aged 6-8 weeks were randomly divided into three groups, with 6 mice in each group, and the grouping process was as follows:
[0126] PBS group: On the 1st day, 5th day, 10th day and 14th day of the experiment, 10 μL of PBS buffer was instilled through the nasal cavity;
[0127] CTB-Sortase A protein 5 μg group: On the 1st day, the 5th day, the 10th day and the 14th day of the experiment, 10 μL CTB-Sortase A protein solution (vaccine solution) was instilled through the nasal cavity respectively, and each dose of these four times 5 μg CTB-Sortase A protein;
[0128] CTB-Sortase A protein 10 μg group: On the 1st day, the 5th day, the 10th day and the 14th day of the experiment, 10 μL CTB-Sortase A protein solution (vaccine solution) was instilled through the nasal cavity respectively. 10 μg CTB-Sortase A protein;
[0129] ...
Embodiment 3
[0131] Example 3. Immunoprotection against Type A Streptococcus infection after nasal cavity inhalation of different doses of CTB-Sortase A protein
[0132] Female BALB / c mice aged 6-8 weeks were randomly divided into four groups, with 6 mice in each group, and the grouping process was as follows:
[0133] PBS group: On the 1st day, 5th day, 10th day and 14th day of the experiment, 10 μL of PBS buffer was instilled through the nasal cavity;
[0134] CTB-Sortase A protein 1 μg group: On the 1st day, the 5th day, the 10th day and the 14th day of the experiment, 10 μL of CTB-Sortase A protein solution (vaccine solution) was instilled through the nasal cavity respectively. 1 μg CTB-Sortase A protein;
[0135] CTB-Sortase A protein 2.5 μg group: 10 μL CTB-Sortase A protein solution (vaccine solution) was instilled through the nasal cavity on the 1st, 5th, 10th and 14th days of the experiment. Both are 2.5 μg CTB-Sortase A protein;
[0136] CTB-Sortase A protein 5 μg group: On th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com