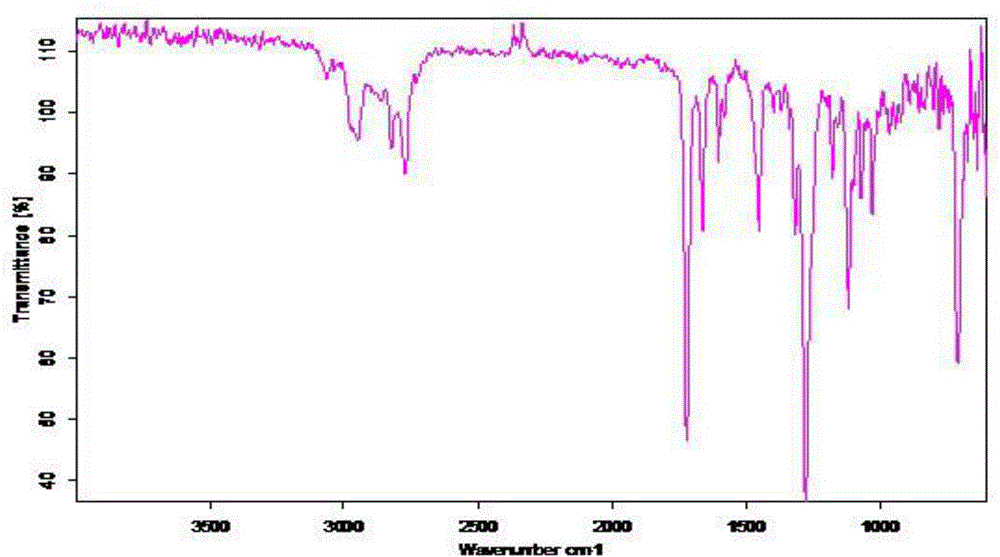
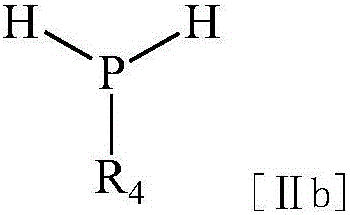
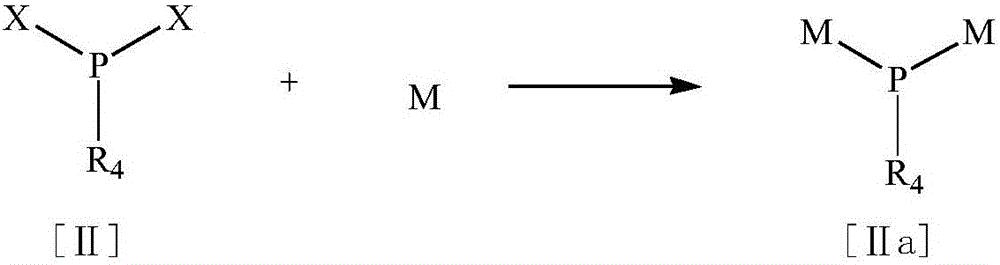Acyl phosphine photoinitiator
A technology of photoinitiator and acylphosphine, which is applied in the field of UV-LED curing photoinitiator and its preparation, can solve the problems of single radiation peak and weak absorption, and achieve the effects of good solubility, strong absorption and fast initiation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the preparation of photoinitiator P6511
[0047]
[0048] a. Metal substitution of P,P-dichloroethoxyphosphine in toluene at 98°C-100°C
[0049] Under an inert gas atmosphere, suspend sodium block (9.2g, 0.4mol) in toluene (500ml) at room temperature, raise the temperature to reflux, start vigorous stirring when the temperature reaches 98°C, disperse into small sodium droplets and add dropwise P,P-dichloroethoxyphosphine (14.7g, 0.1mol) was used for more than one hour, and then heated under reflux for 16h to form a yellow suspension, which was directly carried out to the next step without purification.
[0050] b. Protonation
[0051] At 98°C-100°C, tert-butanol (14.8g 0.2mol) was added dropwise to the above yellow suspension, and the reaction was refluxed until all the sodium was reacted, and the product was directly subjected to the next reaction without purification.
[0052] c. Condensation
[0053] Add p-dimethylaminobenzaldehyde (29.8g, 0.2mol)...
Embodiment 2
[0056] Embodiment 2: the preparation of photoinitiator P6512
[0057]
[0058] a. Metal substitution of P,P-dichlorophenylphosphine in toluene at 98°C-100°C
[0059] Under an inert gas atmosphere, suspend sodium block (9.2g, 0.4mol) in toluene (500ml) at room temperature, raise the temperature to reflux, start vigorous stirring when the temperature reaches 98°C, disperse into small sodium droplets and add dropwise P,P-dichlorophenylphosphine (17.8g, 0.1mol) was used for more than one hour, and then heated under reflux for 16h to form a yellow suspension, which was directly carried out to the next step without purification.
[0060] b. Protonation
[0061] At 98°C-100°C, tert-butanol (14.8g 0.2mol) was added dropwise to the above yellow suspension, and the reaction was refluxed until all the sodium was reacted, and the product was directly subjected to the next reaction without purification.
[0062] c. Condensation
[0063] Add p-dimethylaminobenzaldehyde (29.8g, 0.2mol)...
Embodiment 3
[0066] Embodiment 3: the preparation of photoinitiator P6513
[0067]
[0068] a. Metal substitution of P,P-dichloromethylphosphine in toluene at 98°C-100°C
[0069] Under an inert gas atmosphere, suspend sodium block (9.2g, 0.4mol) in toluene (500ml) at room temperature, raise the temperature to reflux, start vigorous stirring when the temperature reaches 98°C, disperse into small sodium droplets and add dropwise P,P-dichloromethylphosphine (11.6g, 0.1mol) was used for more than one hour, and then heated under reflux for 16h to form a yellow suspension, which was directly carried out to the next step without purification.
[0070] b. Protonation
[0071] At 98°C-100°C, tert-butanol (14.8g 0.2mol) was added dropwise to the above yellow suspension, and the reaction was refluxed until all the sodium was reacted, and the product was directly subjected to the next reaction without purification.
[0072] c. Condensation
[0073] Add p-dimethylaminobenzaldehyde (29.8g, 0.2mol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com