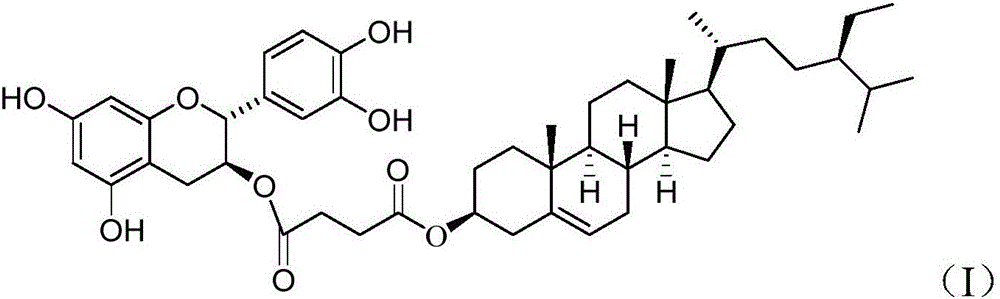
Beta-sitosterol type catechinic acid liposolubility antioxidant and preparation method thereof
A technology of catechin fat and sitosterol type, which is applied in the field of β-sitosterol type catechin molecule and its preparation, can solve the problems of limited practical use range and poor solubility of free phytosterol, and achieve good fat-soluble anti-oxidation Functional, cheap, good antioxidant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] β-sitosterol (414.7mg), succinic anhydride (100.1mg) and triethylamine (151.0mg) were stirred and reacted at room temperature in dimethylformamide for 1 hour, and extracted with ethyl acetate after the reaction was completed, to obtain The product was reacted with tetrapropionylcatechin (514.3mg) and N,N'-dicyclohexylcarbodiimide (412.0mg) at room temperature for 2 hours with stirring, and treated with hydrazine (300.0mg) after the reaction Then extracted with chloroform, and purified by chromatography to obtain 70% of β-sitosterol type catechin.
[0030] Structure determination data: NMR data show the characteristic peaks of 5-9ppm catechin and 1-4ppm β-sitosterol, IR 1731cm -1 The characteristic absorption peaks of ester groups are shown on the left and right, and the mass spectrum shows the molecular ion peak of 809.43 after adding sodium. These data demonstrate the correctness of the structure of the synthesized substance.
Embodiment 2
[0032] β-sitosterol (414.7mg), succinic anhydride (150.1mg) and potassium carbonate (210.0mg) were stirred and reacted at room temperature in dimethylformamide for 1 hour, and extracted with ethyl acetate after the reaction was completed, and the obtained The product reacted with tetrapropionyl catechin (600.3mg) and N,N'-dicyclohexylcarbodiimide (490.0mg) at room temperature for 2 hours with stirring. After the reaction was completed, it was treated with hydrazine (300.0mg) and then Chloroform extraction, chromatographic purification to obtain β-sitosterol type catechin 81%.
Embodiment 3
[0034] β-sitosterol (414.7mg), succinic anhydride (150.1mg) and sodium hydroxide (50.0mg) were stirred and reacted in dimethylformamide at room temperature for 1 hour, and extracted with ethyl acetate after the reaction was completed to obtain The product was reacted with tetrapropionylcatechin (640.3mg) and N,N'-dicyclohexylcarbodiimide (550.0mg) at room temperature for 2 hours with stirring, and treated with hydrazine (300.0mg) after the reaction Then extracted with chloroform and purified by chromatography to obtain 85% of β-sitosterol type catechin.
[0035] Antioxidant activity test: The fat-soluble antioxidant activity of β-sitosterol catechin was tested using the classic liposome model. The results showed that the antioxidant activity of β-sitosterol catechin was 5 times that of catechin. times, 20% higher than the commonly used strong fat-soluble antioxidant TBHQ.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com