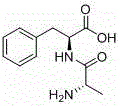
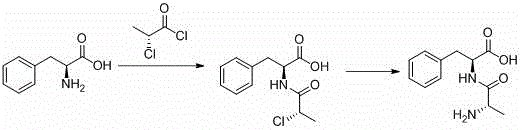
Preparation method for chiral L-alanyl-phenylalanine
A technology of chiral phenylalanine and phenylalanine, applied in the direction of peptides, etc., can solve the problem of unreported L-alanyl-phenylalanine, etc., and achieve easy post-processing, readily available raw materials, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 2-chloropropionyl-L-phenylalanine
[0033] In a 500mL reaction flask, add 165mL of chloroform, 16.5g of L-phenylalanine (0.1mol), 30.3g of triethylamine (0.3mol), and dropwise add 25.4g of D-2-chloropropionyl chloride (0.2mol) at room temperature, The pH value of the reaction is stable between 8-10, and the raw materials are completely reacted after 5 hours of reaction. Then lower the temperature to 1-5°C, adjust the pH to 1-2 with hydrochloric acid, stir for 1 hour, filter with suction, and dry the solid to obtain 23.7 g of product (I), the yield is 93.2%, and the HPLC purity is ≥97%.
Embodiment 2
[0035] Preparation of 2-chloropropionyl-L-phenylalanine
[0036] In a 250mL reaction flask, add 90mL of butyl acetate, 8.25g of L-phenylalanine (0.05mol), 27.6g of potassium carbonate (0.2mol), drop 19g of D-2-chloropropionyl chloride (0.15mol) at room temperature, and react The pH value is between 9-11, and the raw materials are completely reacted after 7 hours of reaction. Cool down to 10-15°C, adjust the pH to 2-3, stir for 1 hour, filter with suction to obtain a solid, and dry to obtain 12.1 g of the product. The yield is 94.5%, and the HPLC purity is 97.7%.
Embodiment 3
[0038] Preparation of 2-chloropropionyl-D-phenylalanine
[0039] In a 2000mL reaction flask, add 543g of water and 132g of sodium hydroxide (3.3mol), stir to dissolve, cool down to room temperature, then add 165g of D-phenylalanine (1mol), dropwise add 381g of D-2- Chloropropionyl chloride (3mol), the reaction pH value is between 9-12, react for 8 hours, the raw materials are completely reacted, separate the liquid to take the water layer, cool down to 1-5°C, adjust the pH to 1-2, stir for 1 hour, and filter to obtain a solid , and dried to obtain 241.6 g of the product 2-chloropropionyl-D-phenylalanine, the yield was 94.5%, and the HPLC purity was 98.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com