Pidotimod orally disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and pidotimod, which is applied in the field of pidotimod orally disintegrating tablets and its preparation, can solve the problems of complicated taking process and inconvenient taking for children, and achieve convenient taking, transportation and taking , reduce the effect of liver first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
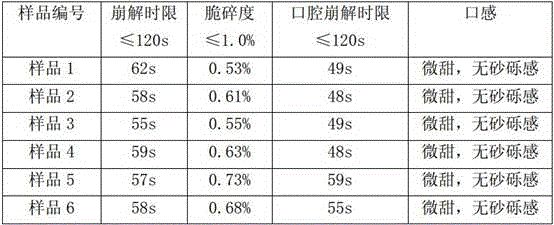
Image
Examples
Embodiment 1
[0022] The preparation process of Pidotimod orally disintegrating tablets (400 / 600mg):
[0023] (1) Pass pidotimod through a 60-mesh sieve;
[0024] (2) Add and mix the sieved raw materials with the prescribed amount of mannitol, microcrystalline cellulose, crospovidone, croscarmellose sodium, and acesulfame potassium;
[0025] (3) Add silicon dioxide and magnesium stearate to the above mixture, mix well and press into tablets with a weight of 600mg and a hardness of 20-40N.
Embodiment 2
[0027] The preparation process of Pidotimod orally disintegrating tablets (200 / 300mg):
[0028] (1) Pass pidotimod through a 60-mesh sieve;
[0029] (2) Add and mix the sieved raw materials with the prescribed amount of mannitol, microcrystalline cellulose, crospovidone, croscarmellose sodium, and acesulfame potassium;
[0030] (3) Add silicon dioxide and magnesium stearate to the above mixture, mix well and press into tablets with a weight of 300mg and a hardness of 20-40N.
Embodiment 3
[0032] Preparation process of pidotimod orally disintegrating tablets (400 / 500mg):
[0033] (1) Pass pidotimod through a 60-mesh sieve;
[0034] (2) Mix the sieved raw materials with the prescribed amount of lactose, microcrystalline cellulose, crospovidone, croscarmellose sodium, acesulfame potassium and aspartame in equal amounts;
[0035] (3) Add silicon dioxide and magnesium stearate to the above mixture, mix well and press into tablets with a weight of 500mg and a hardness of 20-40N.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com