Memantine hydrochloride/donepezil slow-release resin composition and preparation method thereof
A technology of donepezil hydrochloride and memantine hydrochloride, which is applied in the field of memantine hydrochloride/donepezil hydrochloride sustained-release resin composition and its preparation, can solve the problem of inconvenient taking of capsule patients and difficulty in swallowing, and the frequency of taking medicine and high dosage, to improve bioavailability, reduce dosing frequency and dosage, and stabilize the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
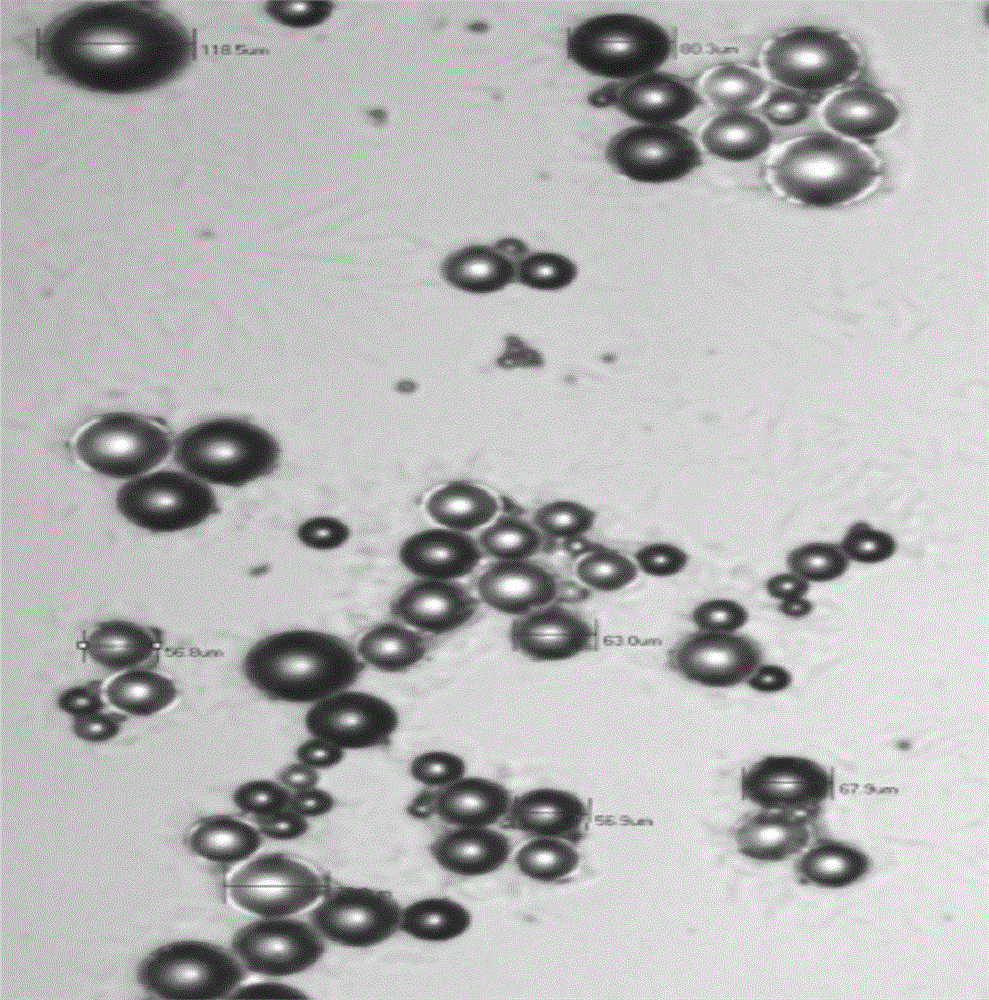
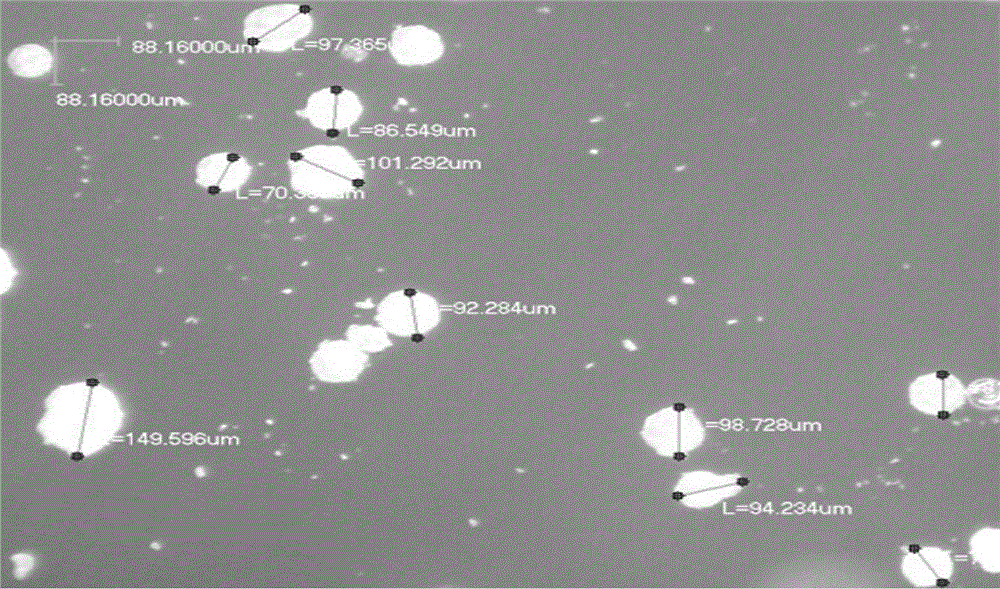
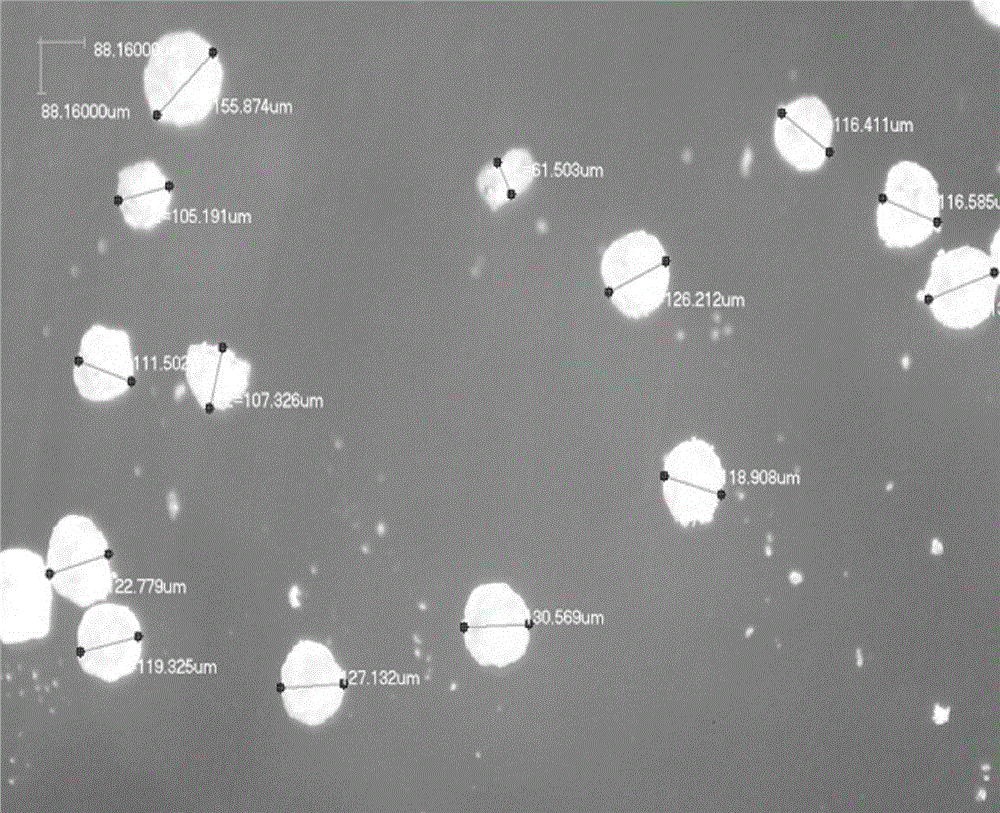
Image
Examples
Embodiment 1
[0039] Example 1 The preparation method of memantine hydrochloride slow-release resin / donepezil resin dry suspension
[0040] First prepare memantine hydrochloride drug-loaded resin and donepezil hydrochloride drug-loaded resin respectively, coat the isolation layer respectively, and then wrap the memantine hydrochloride isolation resin into the sustained-release layer to obtain the memantine hydrochloride sustained-release resin, according to the dosage specifications and dry suspension The characteristics of adding auxiliary materials, mixing evenly, can be.
[0041] (1) Preparation process of memantine hydrochloride drug-loaded resin and donepezil hydrochloride drug-loaded resin
[0042] Prescription of drug-loaded resin: cationic resin (particle size: 0.08-0.20mm) 400g, memantine hydrochloride 180g, donepezil hydrochloride 40g, PEG4000 372g.
[0043] Preparation process of memantine hydrochloride medicinal solution: add 180 g of memantine hydrochloride into 5040 mL of pur...
Embodiment 2
[0057] Example 2 The preparation method of memantine hydrochloride / donepezil hydrochloride dry suspension
[0058] Prepare the memantine hydrochloride / donepezil hydrochloride drug-loaded resin first, coat the isolation layer, and then coat the sustained-release layer to prepare the memantine hydrochloride / donepezil hydrochloride sustained-release resin, add auxiliary materials according to the characteristics of the dry suspension, mix evenly, and then .
[0059] (1) Preparation process of memantine hydrochloride / donepezil hydrochloride drug-loaded resin
[0060] Memantine hydrochloride / donepezil hydrochloride drug-loaded resin composition: cationic resin (particle size: 0.10-0.15mm) 400g, memantine hydrochloride 160g, donepezil hydrochloride 60g, PEG4000 372g.
[0061] Preparation process of memantine hydrochloride medicinal solution: add 160 g of memantine hydrochloride into 4480 mL of purified water under stirring, and dissolve until clear to obtain a drug-loaded memantine...
Embodiment 3
[0077] Example 3 The preparation method of memantine hydrochloride / donepezil hydrochloride dry suspension
[0078] Prepare the memantine hydrochloride / donepezil hydrochloride drug-loaded resin first, coat the isolation layer, and then coat the sustained-release layer to prepare the memantine hydrochloride / donepezil hydrochloride sustained-release resin, add auxiliary materials according to the characteristics of the dry suspension, mix evenly, and then .
[0079] (1) Preparation process of memantine hydrochloride / donepezil hydrochloride drug-loaded resin
[0080] Memantine hydrochloride / donepezil hydrochloride drug-loaded resin composition: cationic resin (particle size: 0.10-0.15mm) 200g, memantine hydrochloride 140g, donepezil hydrochloride 50g, PEG4000 234g.
[0081] Preparation process of the drug-loaded liquid: add 140 g of memantine hydrochloride and 50 g of donepezil hydrochloride into 3920 mL of purified water under stirring, and dissolve until clear to obtain a drug-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com