Preparation method for blue TiO2 catalyst
A catalyst and blue technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve problems such as harsh conditions, complicated processes, and unfavorable large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 1) TiCl 4 Mix with hydrazine hydrate at a molar ratio of 1:8 to obtain TiCl 4 -hydrazine complexes;
[0017] 2) According to the mass ratio of TiCl 4 : water = 1:20, adding deionized water to the complex in (1) above to obtain TiO 2 precursor fluid of
[0018] 3) Put the precursor liquid obtained in 2) into a hydrothermal kettle, react for 20 hours at 180°C, filter with suction, wash with ethanol, and dry to obtain blue TiO 2 catalyst.
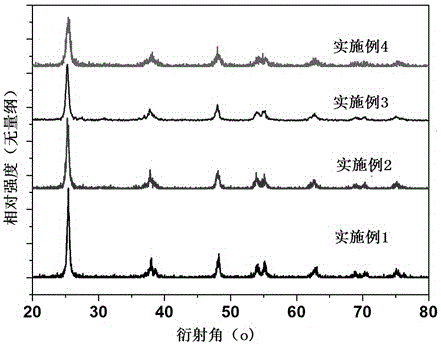
[0019] Utilize German Bruker D8 Advance to carry out XRD spectrogram test to the obtained sample, the result is as follows figure 1 . XRD spectrum and anatase TiO 2 The standard card is the same, indicating that in Example 1, the blue anatase TiO was synthesized by one-step method 2 ; From the broadening of the diffraction peaks, it can be seen that the grain size of the sample is smaller.
[0020] The blue TiO prepared in this example 2 The powder is added to a 20mg / L methyl orange solution at a mass ratio of 0.1g / 100mL. Unde...
Embodiment 2
[0022] 1) TiCl 4 Mix with hydrazine hydrate at a molar ratio of 1:10 to obtain TiCl 4 -hydrazine complexes;
[0023] 2) According to the mass ratio of TiCl 4 : water=1:25, deionized water is added in the complex in above-mentioned (1), obtains the precursor liquid of TiO2;
[0024] 3) Put the precursor liquid obtained in 2) into a hydrothermal kettle, react at 220°C for 16 hours, filter with suction, wash with ethanol, and dry to obtain blue TiO 2 catalyst.
[0025] Utilize German Bruker D8 Advance to carry out XRD spectrogram test to the obtained sample, the result is as follows figure 1 . XRD spectrum and anatase TiO 2 The standard card is the same, indicating that in Example 2, the blue anatase TiO was synthesized by one-step method 2 ; From the broadening of the diffraction peaks, it can be seen that the grain size of the sample is smaller.
[0026] The blue TiO prepared in this example 2 The powder is added to a 20mg / L methyl orange solution at a mass ratio of 0....
Embodiment 3
[0028] 1) TiCl 4 Mix with hydrazine hydrate at a molar ratio of 1:8 to obtain TiCl 4 -hydrazine complexes;
[0029] 2) According to the mass ratio of TiCl 4 : water=1:15, deionized water is added in the complex in above-mentioned (1), obtains the precursor body liquid of TiO2;
[0030] 3) Put the precursor body liquid obtained in (2) into a hydrothermal kettle, react at 220°C for 16 hours, filter with suction, wash with ethanol, and dry to obtain blue TiO 2 catalyst.
[0031] Utilize German Bruker D8 Advance to carry out XRD spectrogram test to the obtained sample, the result is as follows figure 1 . XRD spectrum and anatase TiO 2 The standard card is the same, indicating that in Example 3, the blue anatase TiO was synthesized by one-step method 2 ; From the broadening of the diffraction peaks, it can be seen that the grain size of the sample is smaller.
[0032] The blue TiO prepared in this example 2 The powder is added to a 20mg / L methyl orange solution at a mass r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com