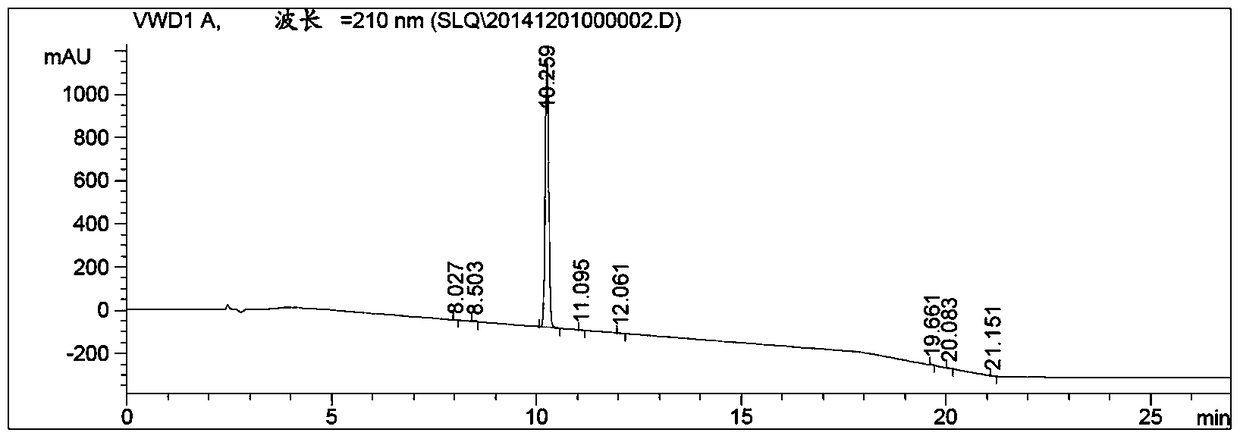
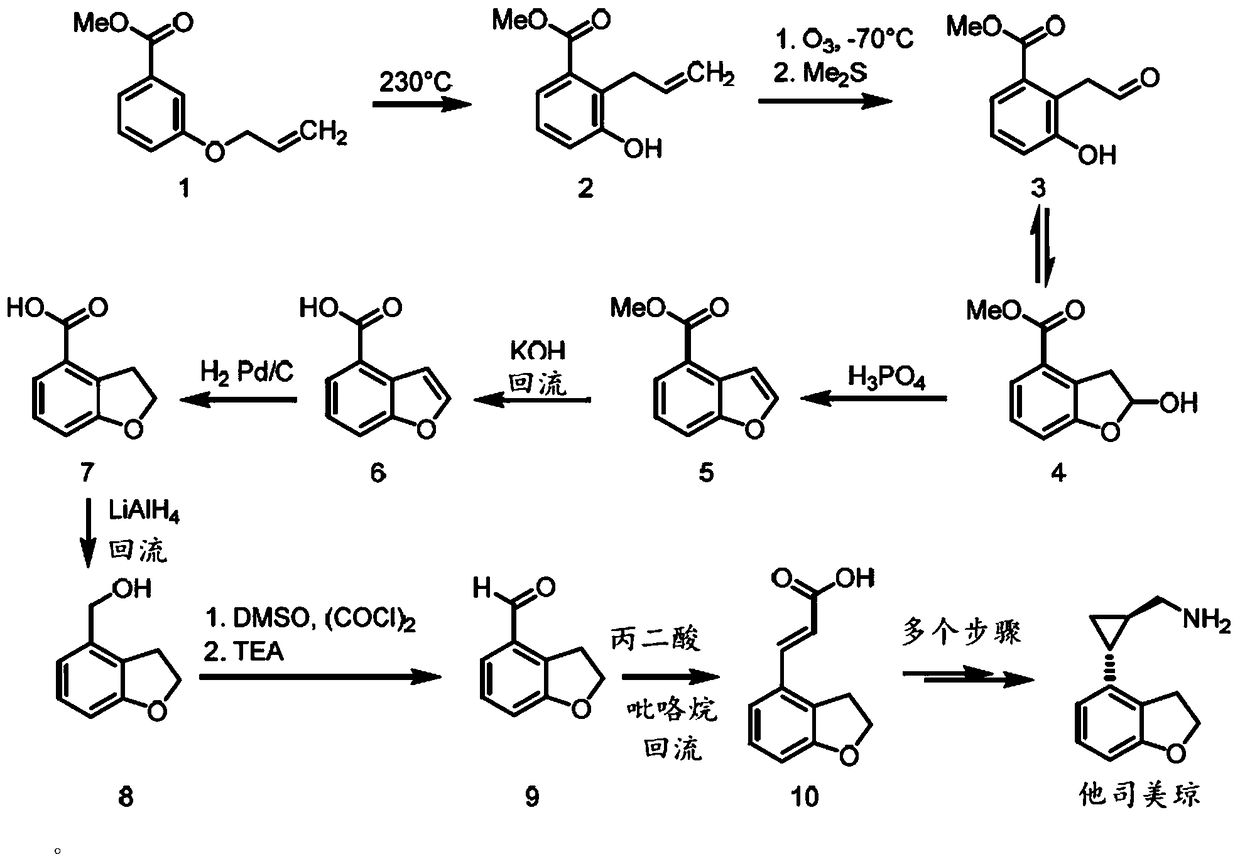
Method for synthesizing 2,3-dihydro-1-benzofuran-4-carbaldehyde
A technology for benzofuran and tetrahydrofuran, which is applied in the field of synthesizing 2,3-dihydro-1-benzofuran-4-carbaldehyde, can solve problems such as low selectivity, danger, and unreportedness, and achieve high yield and selectivity , less by-products, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a method for synthesizing 2,3-dihydro-1-benzofuran-4-carbaldehyde with high conversion rate and good selectivity, which is suitable for industrial scale production. In particular, according to the method of the present invention, DHBFCA can be prepared from 2-(2',6'-dihalophenyl)ethanol (formula (II) ) as the starting substrate is economically and conveniently obtained by Ullman-Bouveault coupling reaction.
[0034] According to one embodiment of the present invention, the present invention provides a method for synthesizing 2,3-dihydro-1-benzofuran-4-carbaldehyde represented by formula (I), comprising: a) adding an alkali metal compound and Cu( I) in the presence of a catalyst, the compound of general formula (II) is subjected to an intramolecular cyclization reaction to produce a compound of general formula (III); and b) the compound of general formula (III) is reacted with magnesium and formula R 3 CONR 1 R 2 The compound of (IV) is reacted ...
preparation example 1
[0054] Preparation Example 1: Preparation of 4-chloro-2,3-dihydrobenzofuran
[0055] Under nitrogen protection, 250 g of 2,6-dichlorophenethyl alcohol, 6.46 g of CuCl and 5.75 g of ethyl acetate were mixed with 7.5 L of tetrahydrofuran. 104.43 g of NaH were added in portions while maintaining the temperature below 70 °C. The resulting mixture was stirred at 70°C for 18 hours. The mass fraction of the starting material in the reaction system was controlled by gas chromatography to 4 dry. The above dried mixture was filtered, and the filter cake was washed with 100 mL of heptane. The filtrates were combined, and 80 g of silica gel was added to the combined filtrates. After fully stirring, filter, and wash the filter cake with 100mL heptane, and then combine the filtrates. The solvent was removed at 40-45°C. 220 g of oily product, ie crude extract of 4-chloro-2,3-dihydrobenzofuran, was obtained with a yield of 109% and a purity of 89%.
[0056] Pass this intermediate throug...
preparation example 2
[0058] Preparation Example 2: Preparation of 2,3-dihydro-1-benzofuran-4-carbaldehyde
[0059] Under nitrogen protection, 46.8g of Mg and 220g of the oil obtained in Preparation Example 1 were mixed in 1.1L of tetrahydrofuran. Add 100 mL of 4-chloro-2,3-dihydrobenzofuran Grignard reagent previously prepared by conventional methods as an initiator. The resulting mixture was heated to reflux and stirred for 18 hours. The reaction is terminated when the mass fraction of the starting materials in the reaction system is detected to be <0.2% by gas chromatography, and the mixture is cooled to a temperature below 20°C.
[0060] In another 3 L flask, 133.51 g of DMF and 660 mL of THF were added. Add the Grignard reagent dropwise to the above step (residual Mg does not transfer with the solution), and keep the temperature below 35°C. After the dropwise addition was complete, the resulting mixture was stirred at room temperature for 3-4 hours. Cool the mixture to a temperature below ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com