Synthesis method of goserelin
A synthetic method and liquid-phase synthesis technology, applied in the field of goserelin synthesis, can solve the problems of high cost, low yield, and expensive price, and achieve the effect of simple and controllable reaction, improved yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
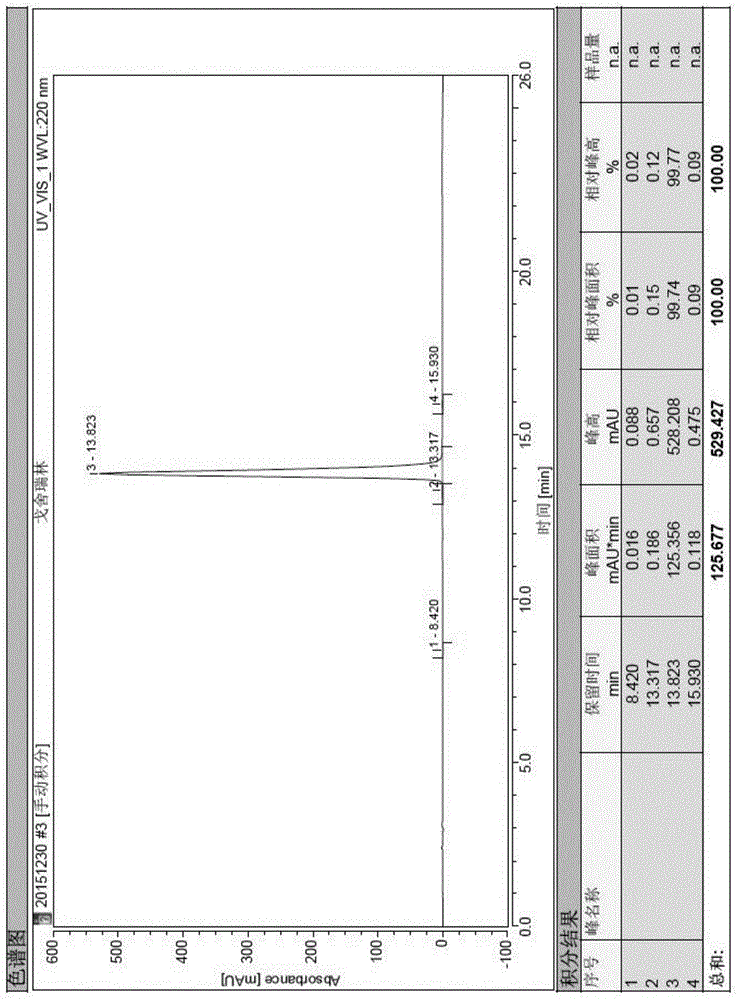
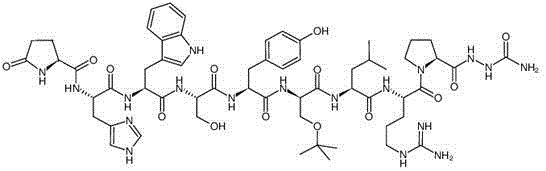
Image
Examples
Embodiment 1
[0033] Example 1, a synthetic method of goserelin: the method adopts solid phase synthesis of 1-5 pentapeptide fragments and then inserts D-Ser(tBu) to form 1-6 hexapeptide fragments; solid phase or liquid phase 7-9 tripeptide fragments are synthesized; 7-9 tripeptides are inserted into semicarbazide in the liquid phase; in the liquid phase, the 1-6 hexapeptide fragments and 7-9 tripeptide fragments are coupled to goserelin to obtain Crude goserelin.
Embodiment 2
[0034] Embodiment 2, a kind of synthetic method of goserelin, concrete steps are as follows:
[0035] (1) Under the action of a condensing agent, Fmoc-Tyr(tBu)-Resin is obtained by condensing Fmoc-Tyr(tBu)-OH and resin;
[0036] (2) Remove Fmoc, and under the action of a condensing agent, sequentially couple the following amino acids: Fmoc-Ser(tBu)-OH, Fmoc-Trp(Boc)-OH, Fmoc-His(Trt)-OH, H-Pyr- OH, to obtain Pyr-His(Trt)-Trp(Boc)-Ser(tBu)-Tyr(tBu)-Resin pentapeptide resin P-1;
[0037] (3) Use a cleavage reagent to cleave the pentapeptide resin to obtain the Pyr-His-Trp-Ser-Tyr pentapeptide fragment P-2;
[0038] (4) Under the action of a condensing agent, P-2 is condensed with H-D-Ser(tBu)-OMe HCl, and then alkaline hydrolyzed to obtain Pyr-His-Trp-Ser-Tyr-D-Ser(tBu), namely P-3 ;
[0039] (5) Under the action of alkali, Fmoc-Pro-OH and CTC Resin are condensed to obtain Fmoc-Pro-CTC Resin;
[0040] (6) Remove Fmoc, under the action of condensing agent, sequentially couple...
experiment example 1
[0052] Experimental example 1, preparation of Fmoc-Pro-CTC Resin
[0053] Take 50.00g of CTC Resin (1.2mmol / g) and place it in a polypeptide reactor, add 400mL of DCM and blow and stir with nitrogen for 30 minutes to fully swell the resin and drain the DCM. Weigh 60.75g of Fmoc-Pro-OH, add DMF 340mL and stir to dissolve, add DIEA 94.5mL, ice bath for 15 minutes, add to the polypeptide reactor, nitrogen blowing and stirring for 2 hours, drain the reaction solution, wash with DMF 3 times, each 400mL each time, 1 minute. Add blocking reagent (anhydrous methanol / DIEA / DCM=1 / 2 / 17 (volume ratio)) to block twice, 400 mL each time, for 10 minutes. Wash with DCM 4 times, 400 mL each time, for 10 minutes. Remove the resin and dry it. Obtained Fmoc-Pro-CTCResin 82.35g. The degree of substitution was 0.73 mmol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com