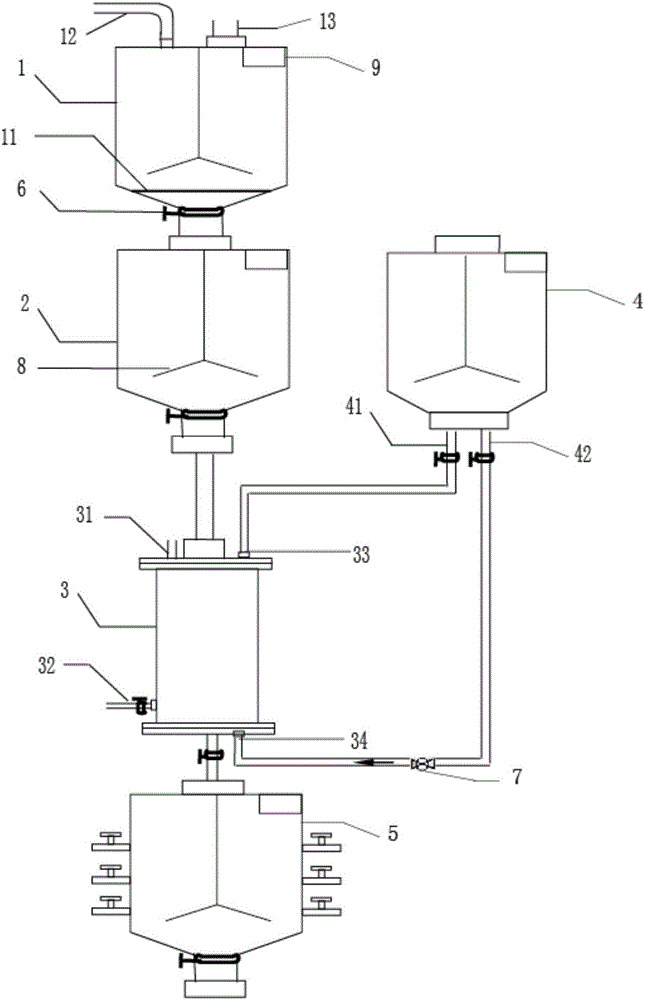
Preparation method of high-titer heparin sodium crude product and production apparatus therefor
A production device and technology of heparin sodium, applied in the biological field, can solve problems such as low biological activity, waste of salt, environmental pollution, etc., and achieve the effects of high product quality, energy saving, and investment reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 is used to improve the reagent composition of crude product heparin sodium purity
[0067] A reagent composition for increasing the potency of heparin sodium, comprising the following reagents:
[0068] Reagent A: a 2% sodium chloride aqueous solution by mass volume percentage;
[0069] Reagent B: 3% sodium chloride aqueous solution by mass volume percentage;
[0070] Reagent C: 4% sodium chloride aqueous solution in mass volume percentage;
[0071] Reagent D: an aqueous solution of 8% sodium chloride by mass volume percentage;
[0072] Reagent E: an aqueous solution of heparin sodium alcohol, where the concentration of ethanol in the aqueous solution of heparin sodium alcohol is 20°.
Embodiment 2
[0073] Embodiment 2 is used to improve the reagent composition of crude product heparin sodium purity
[0074] A reagent composition for increasing the potency of heparin sodium, comprising the following reagents:
[0075] Reagent A: a 5% sodium chloride aqueous solution by mass volume percentage;
[0076] Reagent B: 6% sodium chloride aqueous solution in mass volume percentage;
[0077] Reagent C: a 7% sodium chloride aqueous solution by mass volume percentage;
[0078] Reagent D: a 20% sodium chloride aqueous solution by mass volume percentage;
[0079] Reagent E: an aqueous solution of heparin sodium alcohol, where the concentration of ethanol in the aqueous solution of heparin sodium alcohol is 40°.
Embodiment 3
[0080] Embodiment 3 is used to improve the reagent composition of crude product heparin sodium purity
[0081] A reagent composition for increasing the potency of heparin sodium, comprising the following reagents:
[0082] Reagent A: 4% sodium chloride aqueous solution by mass volume percentage;
[0083] Reagent B: 5% sodium chloride aqueous solution by mass volume percentage;
[0084] Reagent C: a 6% sodium chloride aqueous solution by mass volume percentage;
[0085] Reagent D: a 14.5% sodium chloride aqueous solution by mass volume percentage;
[0086] Reagent E: an aqueous solution of heparin sodium alcohol, where the concentration of ethanol in the aqueous solution of heparin sodium alcohol is 30°.
[0087] The mass volume percentage in the following examples is represented by w / v.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com