Porcine epidemic diarrhea virus attenuated vaccine strain and its culture method and use
A porcine epidemic diarrhea and attenuated vaccine technology, which is applied in the field of porcine epidemic diarrhea virus attenuated vaccine, can solve the problems of poor treatment effect, poor immunogenicity, and difficulty in prevention, so as to prevent porcine epidemic diarrhea and prevent infection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Attenuation of Porcine Epidemic Diarrhea Virus
[0034] 1. Attenuated cultivation of porcine epidemic diarrhea virus
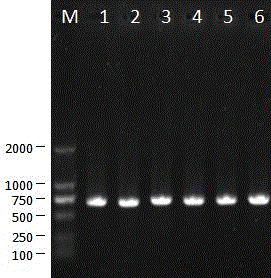
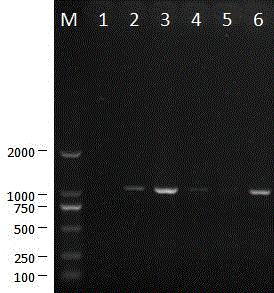
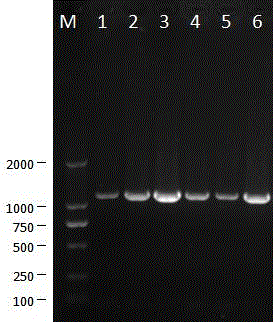
[0035] The isolated porcine epidemic diarrhea virus was continuously passaged on Vero cells for 120 generations. During this process, it was continuously cloned and purified for 3 times, and then continued to be passaged on Vero cells for 150 generations. The 0-, 30-, 50-, 80-, 120- and 150-generation viruses were tested by RT-PCR, and the S gene and ORF3 gene sequences were amplified for analysis. The primers for amplifying the ORF3 gene sequence were: ORF3F5'CTAGACTTCAACCTTACGAA 3', ORF3R 5'TACTAGACCATTATCATTCACT 3', and the fragment size of the amplified ORF3 gene was 734 bp ( Figure 7 ). The amplified S gene sequence is divided into 4 sections for amplification, and the primers are: primer S1F 5'TAGTGATGTTGTGTTAG 3', see SEQ ID No:2; S1R 5'CGCTGCACAGCAGCTC 3', SEQ ID No:3; S2F5'CATACCAGAAGGTTTTAG 3' , see SEQ ID No: 4; S2R 5' GTAATCAAC...
Embodiment 2
[0054] Example 2 Safety Evaluation of Porcine Epidemic Diarrhea Virus Attenuated Strain NB120
[0055] Dilute NBF120 virus to 10 6.0 TCID 50 / ml (10 piglets), 10 piglets at the age of 5 days, were randomly divided into two groups, 5 piglets in the inoculation group, each piglet was orally administered with 2ml, and 5 piglets in the control group were injected with the same dose of DMEM, and observed for 14 days; 5 pregnant sows were given 4ml orally, and 5 sows in the control group were injected with the same dose of DMEM; the sows' food intake, energy, vomiting, diarrhea, abortion and sow litter size were observed.
[0056] The result proves that large-dose oral inoculation of the attenuated strain has no effect on piglets and sows, and there is no incidence of vomiting and diarrhea. The sows did not suffer from reproductive impairment symptoms such as miscarriage and stillbirth, and there was no significant difference in litter size between the experimental group and the c...
Embodiment 3
[0058] Embodiment 3 Porcine epidemic diarrhea virus attenuated strain NB120 immune efficacy evaluation
[0059] One active immunization test
[0060] 40 5-day-old piglets were selected, and the PEDV neutralizing antibody was less than 1:8, and they were divided into ABC three groups on average, AB was the immunization group, and C group was the control group. Group A took PEDV NBF120 1ml orally (virus content 10 5.0 TCID 50 / ml); Group B intramuscular injection of attenuated PEDV vaccine strain CV777 1ml (virus content 10 5.0 TCID 50 / ml); Group C took the same dose of DMEM orally. After 14 days of inoculation, the immunized group and the control group were given porcine epidemic diarrhea virus virulence (10 5.0 TCID 50 / ml) 1ml, observe for 7 days, and count the incidence of each group.
[0061] 14 days after the virus inoculation, the test pigs were all normal, and the challenge protection results of each group are shown in Table 7. It can be seen from the table that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com