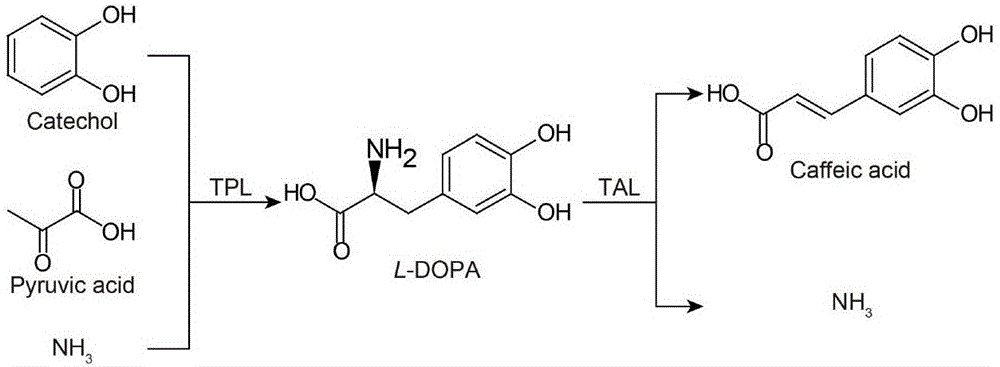
Method for efficient synthesis of caffeic acid with catechol as substrate
A technology of catechol and caffeic acid, which is applied in the field of high-efficiency synthesis of caffeic acid, can solve the problems of not significantly increasing the yield, poor solubility, long production cycle and the like, and achieve the effect of increasing the yield of caffeic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 The construction method of recombinant Escherichia coli
[0036] (I) Construction of EhTPL and RgTAL co-expression vector
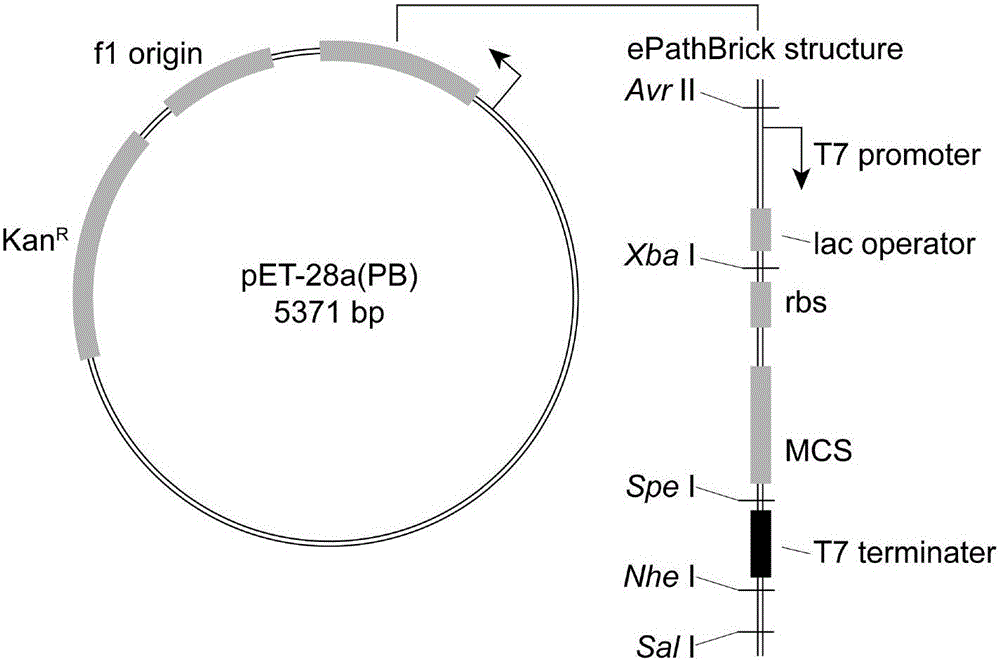
[0037] The genes EhTPL and RgTAL were optimized and synthesized by GenScript Biotechnology Co., Ltd. and cloned into pUC57-Simple. The recombinant plasmids were named pUC57-EhTPL and pUC57-RgTAL respectively. pET28a(PB) is an ePathBrick expression vector constructed on the basis of pET-28a(+), the vector contains homozygous enzymes Avr II, Xba I, Spe I and Nhe I, that is, the downstream Sal I, which can be constructed by ePathBrick strategy. co-expression structure. The structure of pET28a(PB) is as follows figure 2 , the DNA sequence is SEQ ID NO.5. The recombinant vectors pUC57-EhTPL, pUC57-RgTAL and the expression vector pET28a(PB) were digested with restriction enzymes Bam HI / Hind III, respectively, and the uncut products were separated by agarose gel electrophoresis, and the target gene EhTPL (1371bp) was recovered separately....
Embodiment 2
[0047] Example 2 Analysis of the ability of recombinant Escherichia coli strEhTPL to catalyze L-dopa synthesis
[0048] In order to recombine the ability of E. coli strEhTPL to catalyze the synthesis of L-dopa from catechol, the E. coli strCtr containing the empty plasmid pET28a (PB) was used as a blank control, and PBS (50mM, pH 7.0) was used as the reaction medium according to the above transformation method, The reaction was carried out at 37° C. and 220 rpm, and samples were taken at specific time points to determine the synthesis of levodopa.
[0049] The results showed that L-dopa was synthesized in the reaction system catalyzed by strEhTPL, but no L-dopa was detected in the blank control. This result validates the ability of EhTPL to catalyze the synthesis of L-dopa from catechol, and the synthesis cannot proceed spontaneously in the absence of tyrosine benzene lyase. The chromatogram and mass spectrum of levodopa are shown in Figure 4 , where A is the chromatogram o...
Embodiment 3
[0050] Example 3 Analysis of the ability of recombinant Escherichia coli strRgTAL to catalyze caffeic acid synthesis and optimization of substrate concentration
[0051] The recombinant E. coli strRgTAL was cultured, and the expression of RgTAL was induced with 0.5 mM IPTG. The collected cells were washed with 25mL PBS, and resuspended in an equal volume of PBS (50mM, pH 7.0) after centrifugation. The cell concentration was OD. 600 =18±1. At the same time, levodopa with a final concentration of 1 g / L was added as a substrate to carry out the reaction, and the reaction was carried out at 37° C. and a constant temperature shaker at 220 rpm. E. coli strCtr containing the empty plasmid pET-28a(PB) was used as a blank control. Samples were taken at specific time points to determine the synthesis of caffeic acid.
[0052] The results showed that caffeic acid was synthesized in the reaction system catalyzed by strRgTAL, while no caffeic acid was detected in the blank control. Thi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com