Oxcarbazepine dry suspension, and preparation method thereof
A dry suspension, oxcarbazepine hydroxypropyl technology, applied in the field of pharmaceutical preparations, can solve the problems of certain risk of API stability, long drying time of wet granulation, poor material fluidity, etc., to improve bioavailability, The effect of reducing the risk of delamination and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
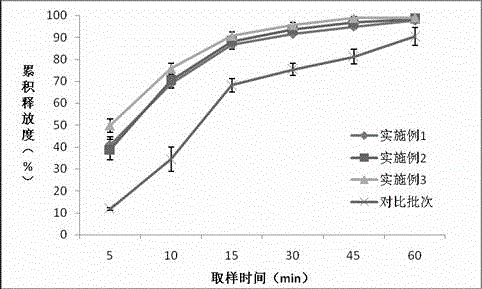
[0021] Example 1: Preparation of oxcarbazepine dry suspension:
[0022]
[0023] Preparation process steps:
[0024] (1) Take 450g of hydroxypropyl-β-cyclodextrin in a colloid mill, add 3L of purified water to fully disperse, then add 300g of oxcarbazepine and grind it into a paste for 2h, freeze-dry to obtain oxcarbazepine hydroxypropyl-β -Cyclodextrin inclusions and solids;
[0025] (2) The prescription amount of mannitol, xanthan gum, titanium dioxide, magnesium stearate and oxcarbazepine hydroxypropyl-β-cyclodextrin package compound is mixed at 10-20rpm for 5-10min in the mixing tank;
[0026] (3) The above mixture is added to the dry granulator, the horizontal screw feeding speed is 25 rpm, the vertical feeding screw speed is 10 rpm, the roller gap is 2 mm, and the screen mesh is 40 meshes to obtain oxcarbazepine dry particles;
[0027] (4) The prescription amount of sodium saccharin, micro-powdered silica gel and lemon flavor are respectively passed through 40 sieves and added to...
Embodiment 2
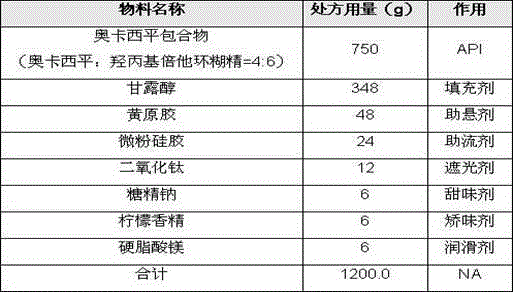
[0029] Example 2: Preparation of oxcarbazepine dry suspension:
[0030] Use the following prescription:
[0031]
[0032] The preparation process steps are the same as in Example 1.
Embodiment 3
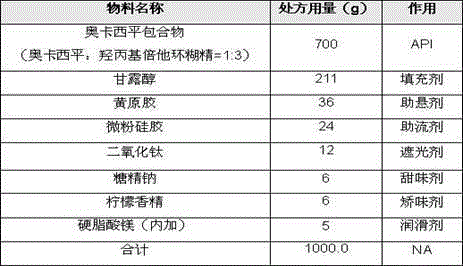
[0033] Example 3: Preparation of oxcarbazepine dry suspension:
[0034] Use the following prescription:
[0035]
[0036] The preparation process steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com