Preparation method and application of non-sulphurized hydrodeoxygenation catalyst
A deoxidation catalyst and non-sulfurization technology, which is applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., to achieve the effect of improving hydrodeoxygenation activity and improving selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
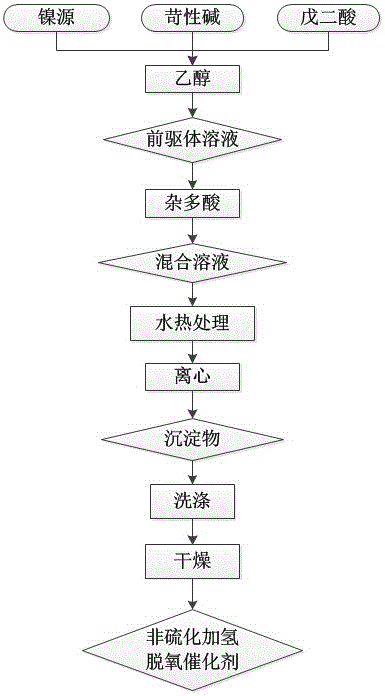
[0020] A preparation method and application of a non-sulfided hydrodeoxygenation catalyst of the present invention, specifically comprising the following steps:
[0021] 1. Weigh the nickel source, caustic alkali, and glutaric acid and add them to the ethanol solution with a volume concentration of 50%, and mix them evenly in a molar ratio of 1.0 : 1.5 :2.0 : 60.0 to obtain a MIL-77 precursor solution.
[0022] The nickel source described in step one is nickel chloride; The caustic alkali is sodium hydroxide.
[0023] 2. Add the heteropoly acid into the solution obtained in step 1 to obtain a mixed solution with a mass concentration of 10%.
[0024] The heteropolyacid described in the second step is phosphotungstic acid.
[0025] 3. After hydrothermally treating the mixed solution obtained in step 2 for 20 hours at 180° C., centrifugation was performed at a speed of 5000 rpm for 10 minutes to obtain a precipitate.
[0026] 4. The precipitate obtained in step 3 was washed 4 t...
Embodiment 2
[0029] A preparation method and application of a non-sulfided hydrodeoxygenation catalyst of the present invention, specifically comprising the following steps:
[0030] 1. Weigh the nickel source, caustic alkali, and glutaric acid and add them to the ethanol solution with a volume concentration of 30%, and mix them uniformly in a molar ratio of 1.0 : 1.5 :2.0 : 60.0 to obtain a MIL-77 precursor solution.
[0031] The nickel source described in step one is nickel nitrate; The caustic alkali is potassium hydroxide.
[0032] 2. Add the heteropoly acid into the solution obtained in step 1 to obtain a mixed solution with a mass concentration of 5%.
[0033] The heteropolyacid described in step two is phosphomolybdic acid.
[0034] 3. After hydrothermally treating the mixed solution obtained in step 2 for 10 h at 220° C., centrifugation was performed at a speed of 6000 rpm for 5 min to obtain a precipitate.
[0035] 4. Wash the precipitate obtained in step 3 three times with deioni...
Embodiment 3
[0038] A preparation method and application of a non-sulfided hydrodeoxygenation catalyst of the present invention, specifically comprising the following steps:
[0039] 1. Weigh the nickel source, caustic alkali, and glutaric acid and add them to the ethanol solution with a volume concentration of 30-70%, and mix them evenly at a molar ratio of 1.0:1.5:2.0:60.0 to obtain a MIL-77 precursor solution.
[0040] The nickel source described in step one is nickel acetate; The caustic alkali is potassium hydroxide.
[0041] 2. Add the heteropoly acid into the solution obtained in step 1 to obtain a mixed solution with a mass concentration of 20%.
[0042] The heteropoly acid described in the second step is silicotungstic acid.
[0043] 3. After hydrothermally treating the mixed solution obtained in step 2 for 30 hours at 120° C., centrifugation was performed at a speed of 4000 rpm for 15 minutes to obtain a precipitate.
[0044] 4. Wash the precipitate obtained in step 3 with deio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com