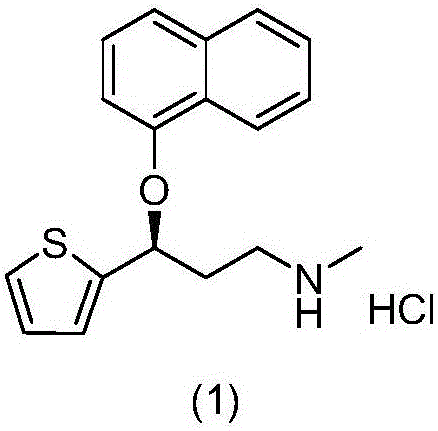
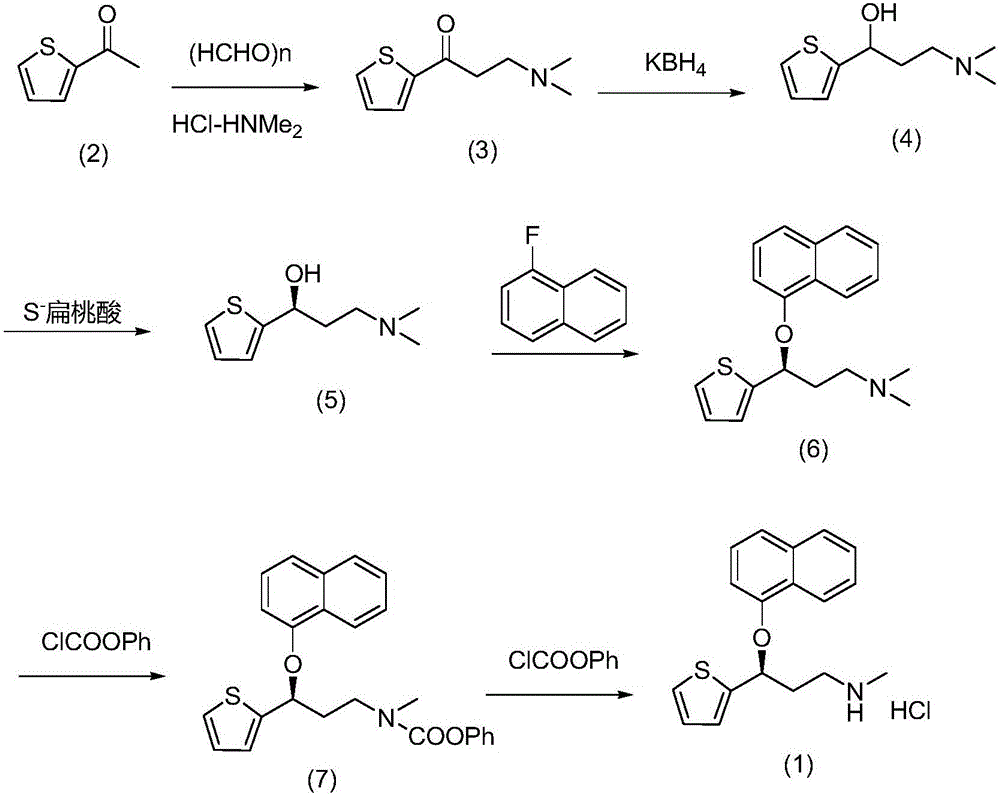
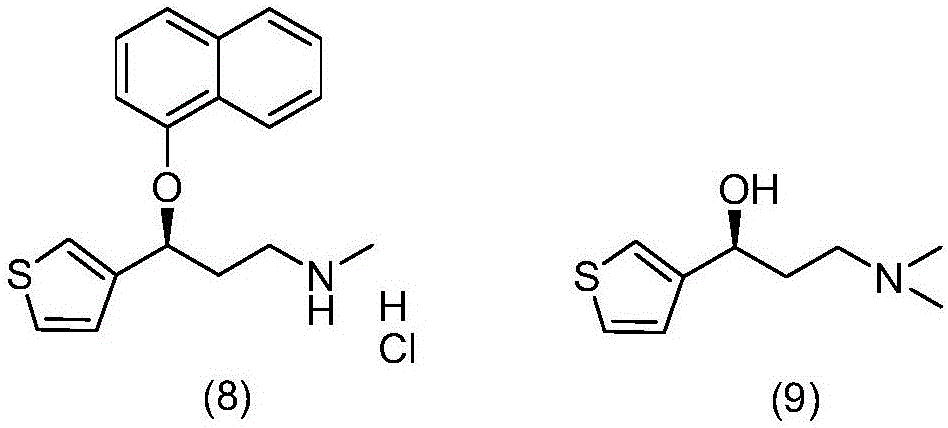
Purification method for preparing high-purity duloxetine hydrochloride intermediate
A technology of duloxetine hydrochloride and purification method, which is applied in the field of medicinal chemistry, can solve the problems of impurity purity less than 0.1%, high quality duloxetine hydrochloride, etc., and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Purification of S-(-)-N, N-dimethyl-3-hydroxyl-3-(2-thiophene) propylamine
[0022] Weigh 30.1g of S-(-)-N,N-dimethyl-3-hydroxy-3-(2-thiophene) propylamine and add it into a 500mL four-neck flask, measure methanol and water with a volume ratio of 1 / 10 Add 293.7g of mixed solvent, heat up the water bath to 72°C, reflux, stir at 5 rpm, stir for 5 minutes, slowly cool down and crystallize by adjusting the temperature control device, control the speed at 6 rpm, and the internal temperature drops to 8 after 140 minutes Suction filtration at ℃, and the filter cake was vacuum-dried at 45 ℃ to obtain 26.8 g of off-white solid. The yield is 89.1%, the HPLC analysis purity is 99.9%, and the purity of the isomer impurity formula (9) is less than 0.03%. The prepared S-(-)-N,N-dimethyl-3-hydroxy-3-(2-thiophene)propylamine is condensed with 1-fluoronaphthalene in the presence of sodium hydride, and finally N-demethylated , refined after salt formation to obtain high-p...
Embodiment 2
[0023] Embodiment 2: Purification of S-(-)-N,N-dimethyl-3-hydroxyl-3-(2-thiophene) propylamine
[0024] Weigh 90.0g of S-(-)-N,N-dimethyl-3-hydroxy-3-(2-thiophene)propylamine and add it into a 1000mL four-neck flask, measure methanol and water with a volume ratio of 1 / 10 Add 881.1g of mixed solvent, raise the temperature of the water bath to 74°C, reflux, stir at 4 rpm, stir for 5 minutes, slowly cool down and crystallize by adjusting the temperature control device, control the speed at 6 rpm, and the internal temperature will drop to 9 after 150 minutes. Suction filtration at ℃, and the filter cake was vacuum-dried at 45 ℃ to obtain 81.9 g of off-white solid. The yield is 91.0%, the HPLC analysis purity is 99.9%, and the purity of isomer impurity formula (9) is less than 0.03%. The prepared S-(-)-N,N-dimethyl-3-hydroxy-3-(2-thiophene)propylamine is condensed with 1-fluoronaphthalene in the presence of sodium hydride, and finally N-demethylated , refined after salt formation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com