Recombinant protein and medicine composition and application thereof
A recombinant protein and composition technology, applied in the field of biomedicine, can solve the problems of low immunogenicity and inability to induce strong cellular immunity, and achieve strong immunogenicity, good application prospects, and the effect of treating cervical cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: DNA structure and plasmid construction
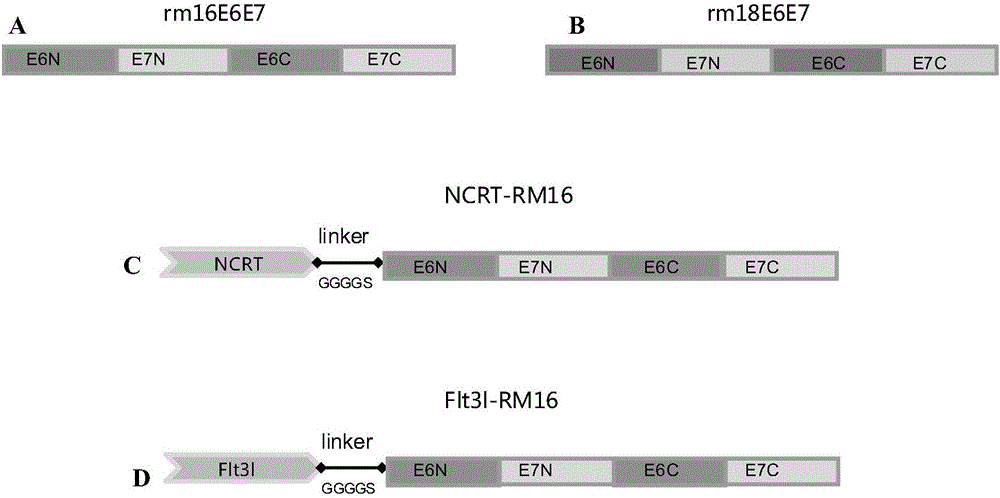
[0107] E6 / E7 protein of human papillomavirus (HPV) 16 / 18 type is the main oncogenic protein with transforming activity. point mutation.
[0108] In HPV16 type E6, the 47th phenylalanine (F) is mutated to arginine (R), the 50th leucine (L) is mutated to glycine (G), and the 63rd cysteine ( C) mutation to glycine (G), cysteine (C) at position 106 to arginine (R); tyrosine (Y) at position 23 in HPV16 E7 was mutated to glycine (G), Cysteine (C) at position 24 is mutated to glycine (G), tyrosine (Y) at position 25 is mutated to glycine (G), cysteine (C) at position 58 is mutated to glycine (G) ), the cysteine (C) at position 91 is mutated to glycine (G).
[0109] In HPV18 type E6, the 49th phenylalanine (F) is mutated to arginine (R), the 52nd leucine (L) is mutated to glycine (G), and the 65th cysteine ( C) mutation to glycine (G), cysteine (C) at position 108 to glycine (G); leucine (L) at position 26...
Embodiment 2
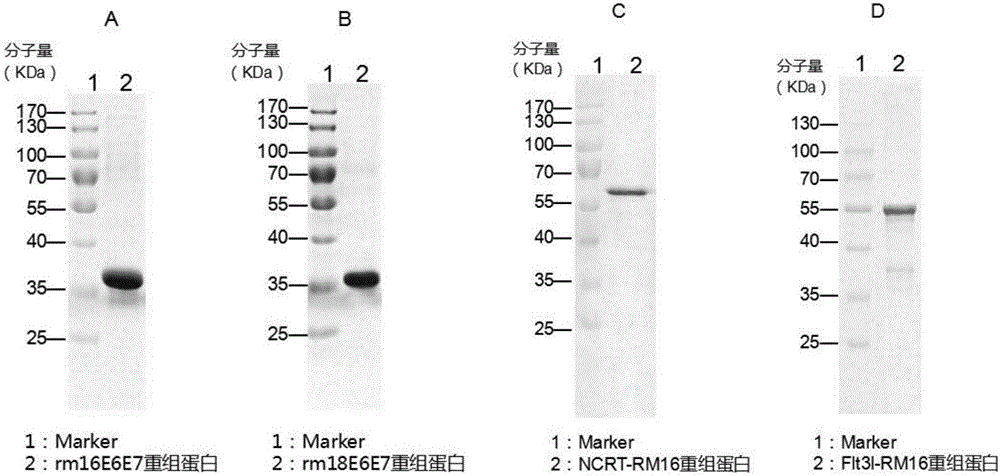
[0114] Example 2: Expression and preparation of recombinant protein in Escherichia coli
[0115] The pET28a-Flt3l-RM16 recombinant expression vector was transformed into BL21 (DE3) Escherichia coli competent, coated with kana resistance plate, and the Escherichia coli expressing the recombinant protein was obtained by colony PCR identification. The engineered bacteria (Escherichia coli successfully expressing Flt3l-RM16 recombinant protein) were expanded in LB medium at 37°C, and 0.4mM IPTG was added when OD600=0.6-0.8, and induced at 16°C for 16h. After the induction was completed, the cells were collected by centrifugation at 7500 g for 5 minutes, and the cells were washed twice with PBS (pH 7.4). Take 20g of engineered bacteria and resuspend them in 100ml PBS (PH7.4) and add PMSF (Beyotime) and EDTA (Sinopharm Group) with a final concentration of 1mM, break the bacteria once with a high pressure homogenizer at 1000bar, collect the broken bacteria solution and centrifuge at ...
Embodiment 3
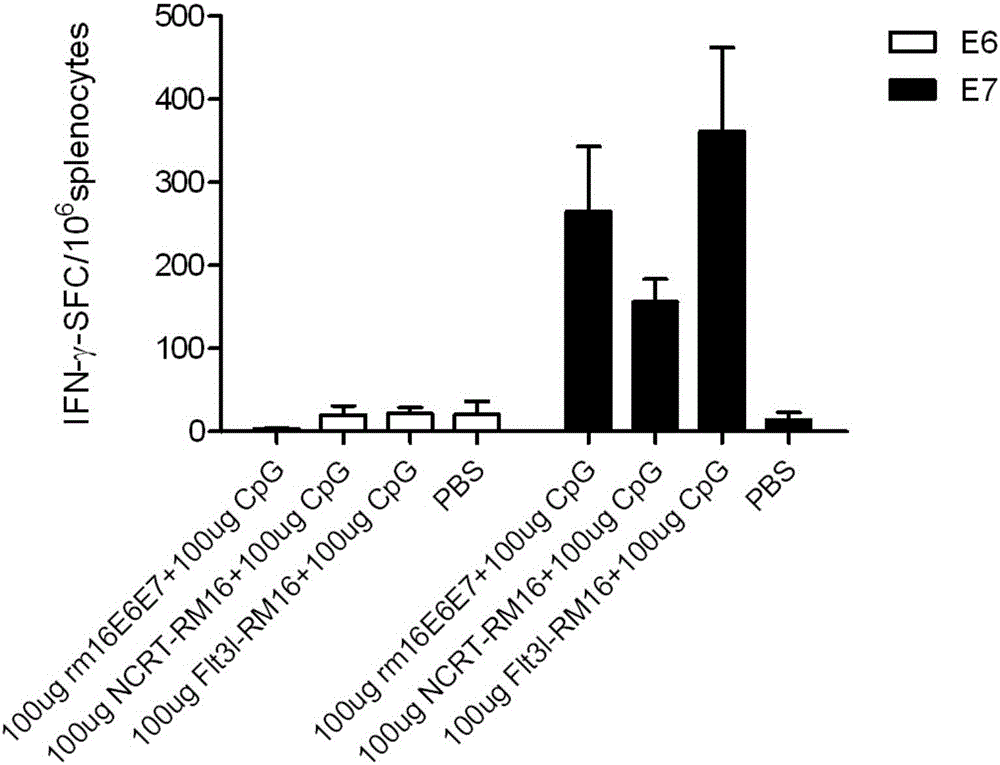
[0119] Embodiment 3: ELISPOT detection cellular immunity level
[0120] Since rm16E6E7 and rm18E6E7; Flt3l-RM16, NCRT-RM16 are similar in structure and function to Flt3l-RM18, NCRT-RM18, therefore, the following Examples 3-6 use rm16E6E7, Flt3l-RM16, NCRT-RM16 as examples.
[0121] Preparation of CpG-ODN: The sequence of CpG-ODN used is shown in Table 1. Utilize solid-phase phosphoramidite triester method chemical synthesis to prepare CpG-ODN, start from 3' end, 1) deprotection group: first use trichloroacetic acid to remove the protection group DMT (two methoxytrityl) to obtain a free 5' hydroxyl group for the next condensation reaction; 2) activation: the phosphoramidite-protected nucleoside monomer is mixed with tetrazolium activator and enters the synthesis column , forming the active intermediate of phosphoramidite tetrazole, which undergoes a condensation reaction with the nucleotide of the deprotected group on CpG; 3) connection: the active intermediate of phosphoramid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com