Organic gel material and preparation method thereof
A technology of organogel and xerogel, which is applied in the field of multiple stimuli responsiveness, preparation of organogel, and synthesis of small molecule gelling factor 4-phenyl)-pyrenecarboylhydrazone, which can solve synthesis difficulties and functional Complicated material structure and other issues, to achieve the effect of convenient purification, easy recycling, and non-toxic recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Synthesis of Gelation Factor and Preparation of Organic Gel Material
[0026] The synthetic steps of gel factor: Weigh 2.0g (0.0051mol) of 3,4,-octyloxybenzoic hydrazide and 1.179g (0.0051mol) of 1-pyrene formaldehyde, put it into a 250mL Erlenmeyer flask, Add 100 mL of absolute ethanol as a solvent, heat to reflux for 6 hours, cool to room temperature after the reaction, filter and dry to obtain a yellow solid. The crude product was recrystallized twice from absolute ethanol. 2.7 g of pure sample 4-(3,4-(octyloxy)phenyl)-pyrenecarboylhydrazone was obtained, with a yield of 87.6%.
[0027] The reaction formula is as follows:
[0028]
[0029] Molecular structure characterization data of materials: 1 H NMR(300MHz,DMSO-d6),(ppm,from TMS):11.94(s,1H),9.645(s,1H),8.753(s,4H),8.20-8.16(d,1H),7.655-7.58 (m,4H),7.30(s,2H),4.10-3.96(m,6H),1.782-1.70(m,6H),1.485(s,6H),1.281(s,24H),0.87(s,9H ).FT-IR(KBr,pellet,cm -1 ): 3433,3170,3065,3034,2926,2857,1675,1637,16...
Embodiment 2
[0031] Example 2 Photoresponse of organogels
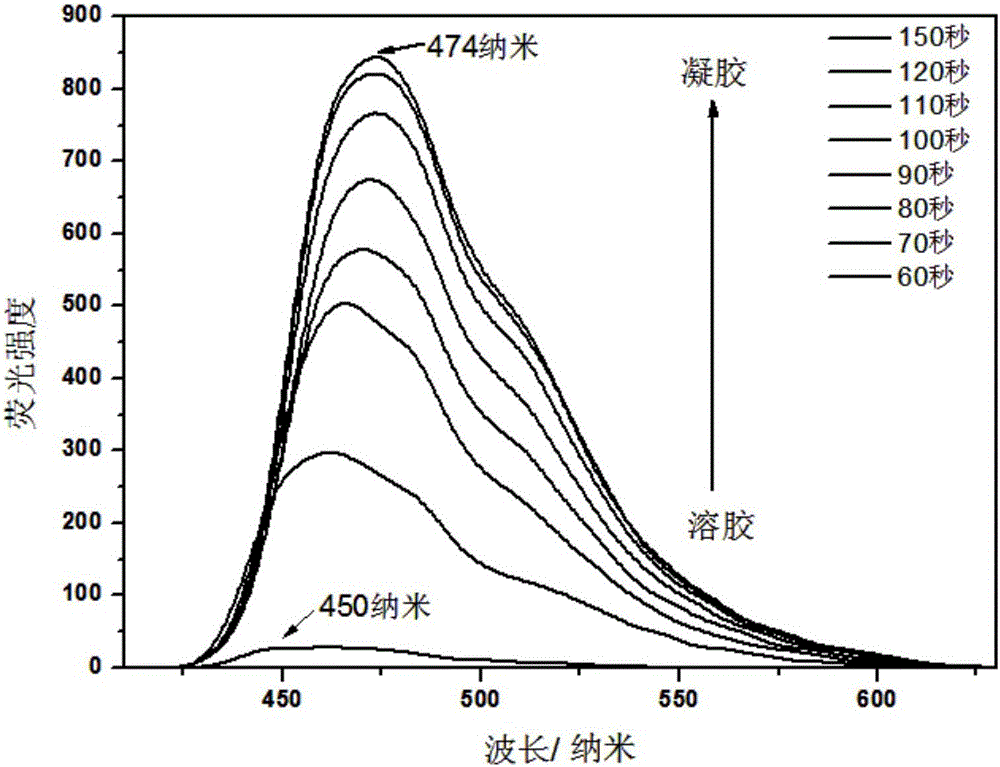
[0032] The gelling factor of the present invention exhibits aggregation-induced fluorescence enhancement during the gelation process, such as figure 1 .
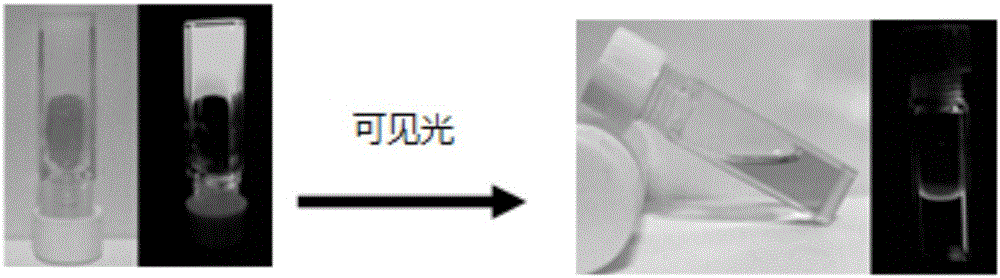
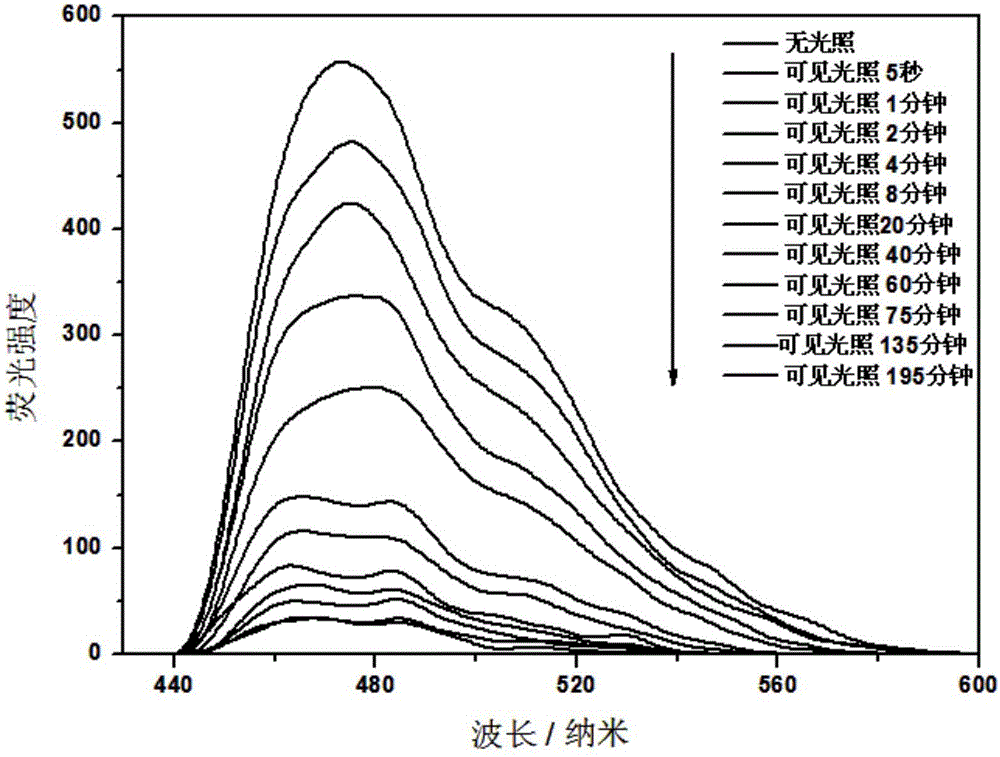
[0033] Photoresponse of the organogel: The organogel was irradiated with visible light, and the organogel transformed into a clear tan solution after a certain period of time. The organogel with a concentration of 5.91mg / mL needs to undergo a gel-sol transition after 195min of light; and with the increase of the light time, the fluorescence intensity is obviously weakened, and after the gel-sol transition occurs, the solution basically does not emit light (see figure 2 and image 3 ).
Embodiment 3
[0034] Example 3 Preparation method of xerogel and force response of xerogel
[0035] The organogel obtained in Example 1 was put into a freeze dryer and dried for 40 hours to remove the dimethyl sulfoxide solvent to obtain a xerogel.
[0036] Such as Figure 4 , the color change of the mechanofluorochromic material (xerogel) before and after grinding seen under a 365nm ultraviolet lamp. The xerogel emits blue fluorescence, and when the xerogel is manually and fully rubbed with the rod of a mortar, the fluorescence of the solid changes from blue to green. However, the ground sample was heated at 130°C for 30 minutes and then observed under a UV lamp, and it was found that it returned to the original blue color.
[0037] Depend on Figure 5The fluorescence spectrum shows that the emission peak of the dry gel without external grinding is around 457nm, corresponding to blue fluorescence; the emission peak of the solid after grinding with a mortar is red-shifted to 514nm, corre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com