Asenapine tablets and preparation method thereof
A technology of asenapine tablets and flat tablets, which is applied in the field of new atypical antipsychotic preparations, can solve the problems of high manufacturing price and inconvenient administration for mental patients, and achieve the effect of improving the dissolution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
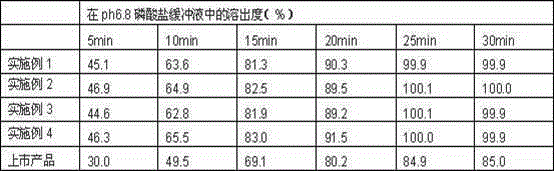
Examples
Embodiment 1
[0022] Asenapine tablet of the present embodiment and preparation method, its each component is:
[0023] 1000 tablets Composition g
[0024] Asenapine 10
[0025] Mannitol 96
[0026] Croscarmellose Sodium 24
[0027] Sodium Lauryl Sulfate 2.5
[0028] Stevioside 1
[0029] Talc v 1.5
[0030] Total 135
[0031] The steps are:
[0032] 1. Micronize 100g of asenapine and 133g of mannitol together, and control the particle size to 0.5-5um;
[0033] 2. Dry and mix 36g of croscarmellose sodium and 2.9g of sodium lauryl sulfate through a 100-mesh sieve for 5 minutes;
[0034] 3. The fine powder obtained in the previous step and 1 / 2 of the disintegrating agent are made into a soft material with an aqueous ethanol solution, and granulated with a 20-mesh sieve;
[0035] 4. Dry the prepared granules at 50°C and sieve with 18 meshes;
[0036] 5. Add the remaining 1 / 2 of the disintegrating agent and 1g of stevioside to the dry granules and mix for 5 minutes, then add 2.1g of t...
Embodiment 2
[0038] Asenapine tablet of the present embodiment and preparation method, its each component is:
[0039] 1000 tablets Composition g
[0040] Asenapine 5
[0041] Lactose 100
[0042] Sodium carboxymethyl starch 16
[0043] Sodium Lauryl Sulfate 1.8
[0044] Aspartan 1.2
[0046] Total 125 The steps are:
[0047] 1. Micronize 100g of asenapine and 154g of lactose together, and control the particle size to 1-5um;
[0048] 2. Dry and mix 32g sodium carboxymethyl starch and 1.8g sodium lauryl sulfate through an 80-mesh sieve for 5 minutes;
[0049] 3. The fine powder obtained in the previous step and 1 / 2 of the disintegrating agent are made into a soft material with an aqueous ethanol solution, and granulated with a 20-mesh sieve;
[0050] 4. Dry the prepared granules at 60°C, and sieve with 18 meshes;
[0051] 5. Add the remaining 1 / 2 of the disintegrating agent and 1.2g of aspartame to the dry granules and mix for 10 minutes, then add 1g o...
Embodiment 3
[0052] Embodiment 3 The asenapine tablet of the present embodiment and preparation method, its each composition is:
[0053] 1000 tablets Composition g
[0054] Asenapine 10
[0055] Lactose 125
[0056] Low-substituted hydroxypropyl cellulose 32
[0057] Sodium Lauryl Sulfate 1
[0058] Aspartan 1.1
[0060] Total 170
[0061] The steps are:
[0062] 1. Micronize 100g of asenapine and 137g of lactose together, and control the particle size to 2-8um;
[0063] 2. Dry and mix 28g of low-substituted hydroxypropyl cellulose and 2g of sodium lauryl sulfate through an 80-mesh sieve for 5 minutes;
[0064] 3. The fine powder obtained in the previous step and 1 / 2 of the disintegrating agent are made into a soft material with an aqueous ethanol solution, and granulated with a 20-mesh sieve;
[0065] 4. Dry the prepared granules at 70°C and sieve with 18 meshes;
[0066] 5. Add the remaining 1 / 2 of the disintegrating agent and 1.2g of aspartame ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com