Nanometer medicine-loaded micelle, nanometer anticancer medicine, methods for preparing nanometer medicine-loaded micelle and nanometer anticancer medicine and application of nanometer medicine-loaded micelle and nanometer anticancer medicine
A technology of nano-drug loading and anti-cancer drugs, which is applied in drug combination, drug delivery, and pharmaceutical formulations. Enhanced thermal effect, increased water solubility, enhanced therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] In this example, nano drug-loaded micelles were prepared by the following method, as follows:
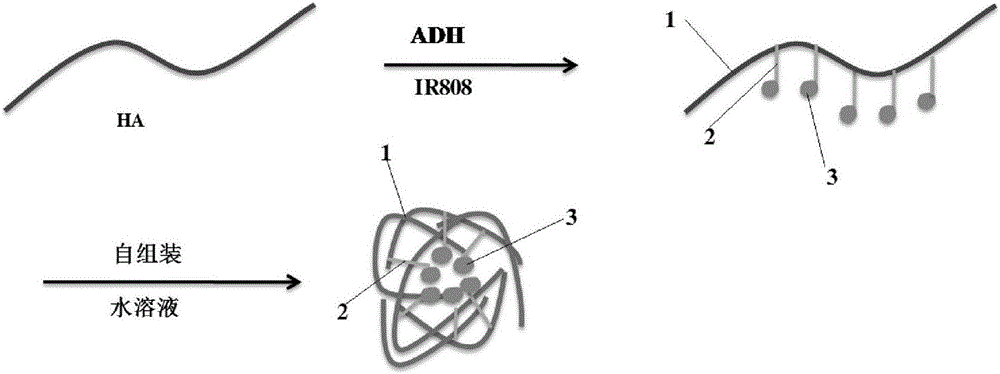
[0060] (1) 15g hyaluronic acid (HA, M W 5K) was dissolved in 5 L of deionized water, 25 g of adipic dihydrazide (ADH) was added to the aqueous solution, and the pH value was adjusted to 4.5 with 0.1 M hydrochloric acid solution under rapid stirring. 15 g of EDC was added to the above reaction solution, and the pH value was adjusted to 4.5 with 0.1 M hydrochloric acid solution, and then reacted at 25° C. for 1 h to obtain a white solid HA-ADH.
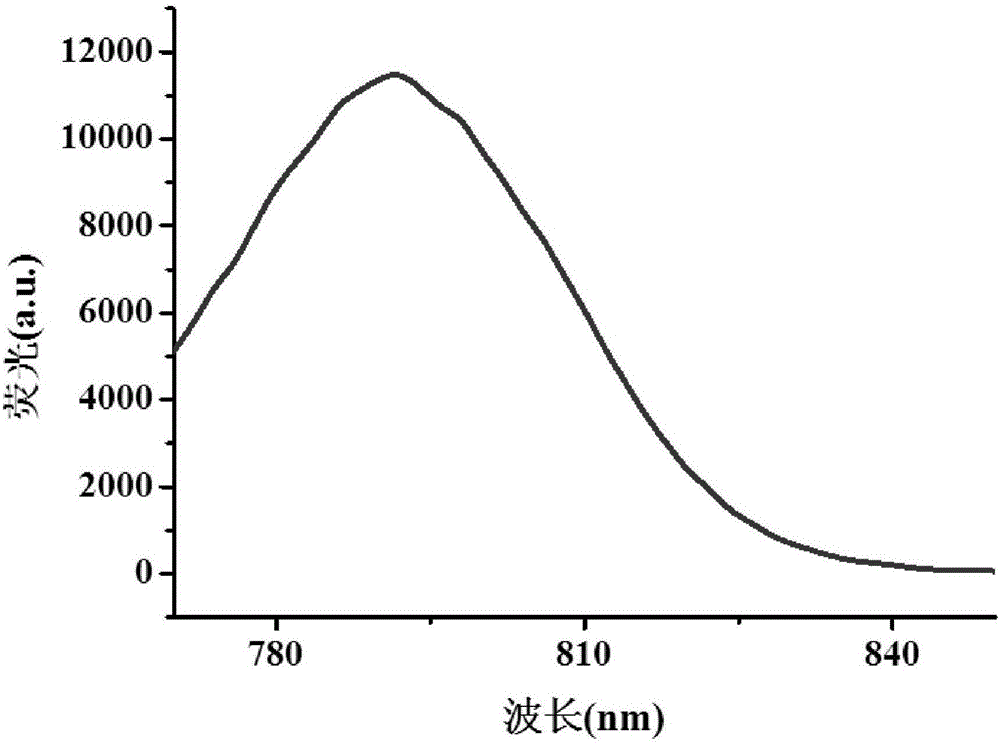
[0061] (2) Dissolve 8g HA-ADH in 2L formamide, activate 25g heptamethine cyanine dye (IR808) with EDC and NHS and dissolve it in a mixed solution of tetrahydrofuran and water, and add the mixed solution dropwise to the above In the formamide solution of HA-ADH, react at 25°C for 24h after the dropwise addition.
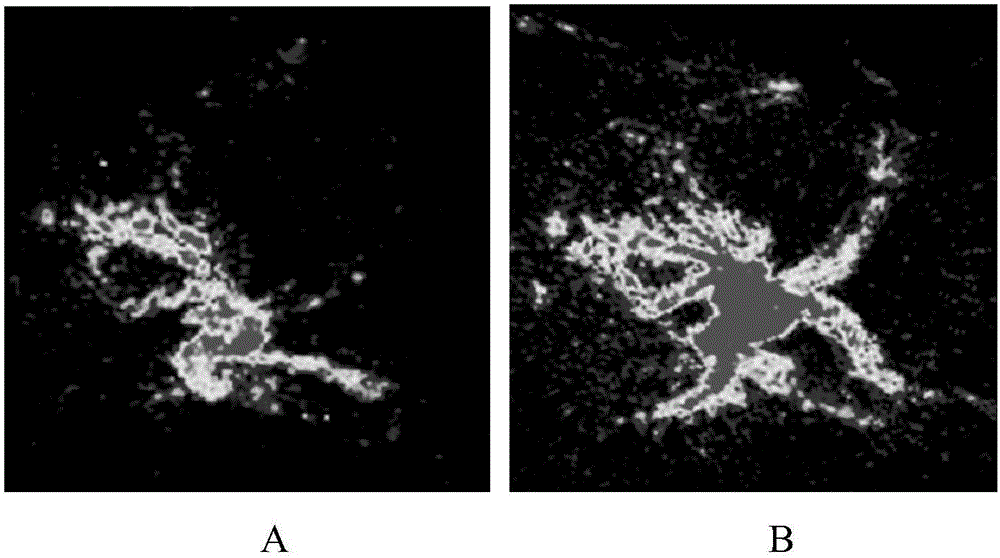
[0062] (3) The reaction solution is filtered under reduced pressure to remove large insoluble matter. The filtrate was pl...
Embodiment 2
[0064] In this example, nano drug-loaded micelles were prepared by the following method, as follows:
[0065] (1) 15g hyaluronic acid (HA, M W 10K) was dissolved in 5 L of deionized water, 45 g of adipic dihydrazide (ADH) was added to the aqueous solution, and the pH value was adjusted to 4.8 with 0.1 M hydrochloric acid solution under rapid stirring. Add 25 g of EDC to the above reaction solution, adjust the pH value to 4.8 with 0.1 M hydrochloric acid solution, and react at 30° C. for 0.5 h to obtain HA-ADH as a white solid.
[0066] (2) Dissolve 10g HA-ADH in 2L formamide, activate 40g heptamethine cyanine dye (IR808) with EDC and NHS and dissolve it in a mixed solution of tetrahydrofuran and water, and add the mixed solution dropwise to the above In the formamide solution of HA-ADH, react at 30°C for 24h after the dropwise addition.
[0067] (3) The reaction solution is filtered under reduced pressure to remove large insoluble matter. The filtrate was placed in a cellul...
Embodiment 3
[0069] In this example, nano drug-loaded micelles were prepared by the following method, as follows:
[0070] (1) 15g hyaluronic acid solution (HA, M W 10K) in 5L of deionized water, add 60g of adipic dihydrazide (adipate dihydrazide ADH) to its aqueous solution, and adjust the pH value to 4.6 with 0.1M hydrochloric acid solution under rapid stirring. Add 35 g of EDC to the above reaction solution, adjust the pH value to 4.6 with 0.1 M hydrochloric acid solution, and react at 18° C. for 5 h to obtain white solid HA-ADH.
[0071] (2) Dissolve 10g HA-ADH in 2L formamide, activate 60g heptamethine cyanine dye (IR808) with EDC and NHS, dissolve it in the formamide solution, and add the mixture dropwise to the above HA-ADH In the formamide solution, react at 20°C for 30h after the dropwise addition is complete.
[0072] (3) The reaction solution is filtered under reduced pressure to remove large insoluble matter. The filtrate was placed in a cellulose dialysis bag, and dialyzed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com