Solid catalyst for dimethyl carbonate and oxamide co-production process and preparation method of solid catalyst
A technology of dimethyl carbonate and solid catalyst, which is applied in the direction of carbonate/haloformate preparation, carboxylic acid amide preparation, chemical instruments and methods, etc. It can solve the problem that the proportion of active components and the loading method cannot meet the co-production reaction Needs and other issues, to achieve the effect of adjusting acidity and alkalinity, rational distribution, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] A. Weigh 11.8g copper nitrate and dissolve it in 20ml deionized water to prepare Cu salt solution.
[0019] B. Weigh 1.5g of calcium nitrate, 8.8g of indium nitrate and 3.8g of cadmium nitrate and dissolve them in 20ml of deionized water to prepare a mixed salt solution.
[0020] C. After uniformly mixing the salt solution prepared in steps A and B, slowly drop 20ml of urea aqueous solution with a concentration of 5mol / L under continuous stirring at 50°C, and after the addition is completed, raise the temperature to 100°C and continue stirring for 12 hours. After cooling down, it was filtered to obtain a colloidal precipitate.
[0021] D. Mix the colloidal precipitate obtained in step C with methanol, and crystallize at 200° C. for 24 hours. After cooling down to room temperature, the precipitate was taken out and filtered, then dried in an oven at 120°C for 12 hours, then placed in a muffle furnace for 3 hours at 650°C, and cooled to room temperature to obtain the cat...
Embodiment 2
[0024] The same as the preparation method of Example 1, the difference lies in step B: 12g of calcium nitrate and 16.6g of cadmium nitrate were weighed and dissolved in 3.7ml of deionized water to prepare a mixed salt solution. The catalyst CuO / CaO-CdO is prepared, wherein CuO accounts for 50% of the total mass of the catalyst, CaO accounts for 40% of the total mass of the catalyst, and CdO accounts for 10% of the total mass of the catalyst.
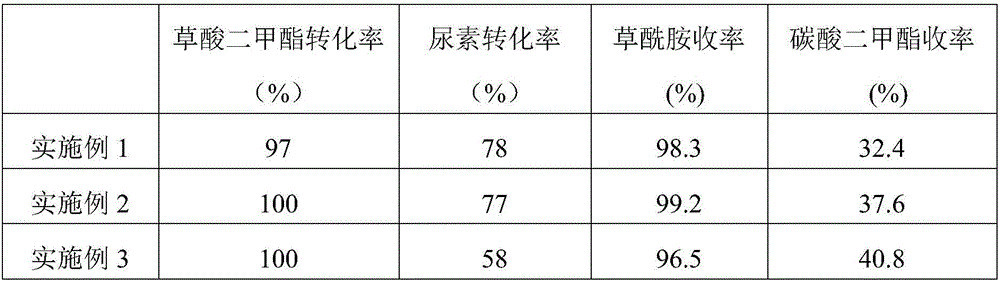
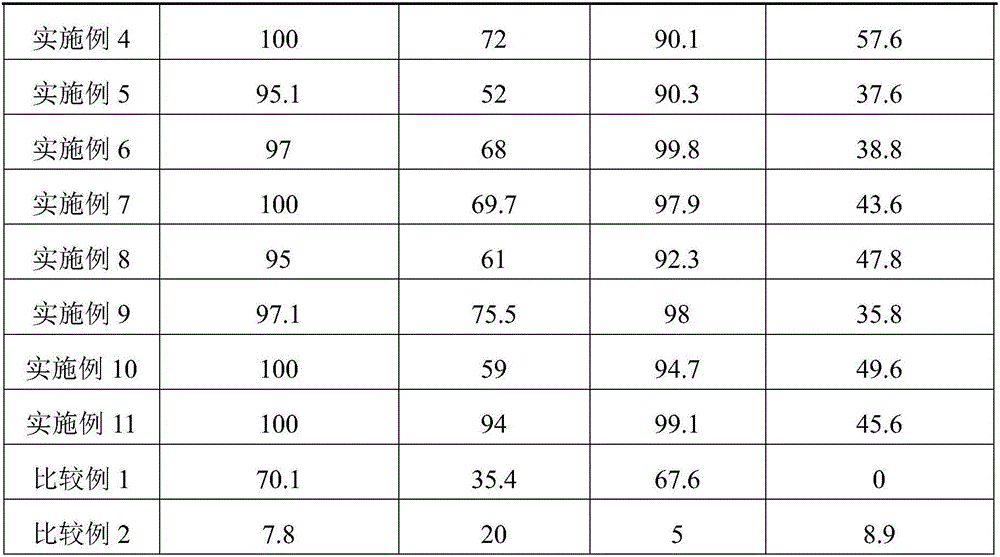
[0025] With the catalyst evaluation method of Example 1, the product analysis results are shown in Table 1.
Embodiment 3
[0027] The same as the preparation method of Example 1, the difference lies in step B: 8.8g of indium nitrate and 3.7g of cadmium nitrate were weighed and dissolved in 23ml of deionized water to prepare a mixed salt solution. Prepared catalyst CuO / In 2 o 3 -CdO, where CuO accounts for 50% of the total mass of the catalyst, In 2 o 3 It accounts for 40% of the total mass of the catalyst, and CdO accounts for 10% of the total mass of the catalyst. With the catalyst evaluation method of Example 1, the product analysis results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com