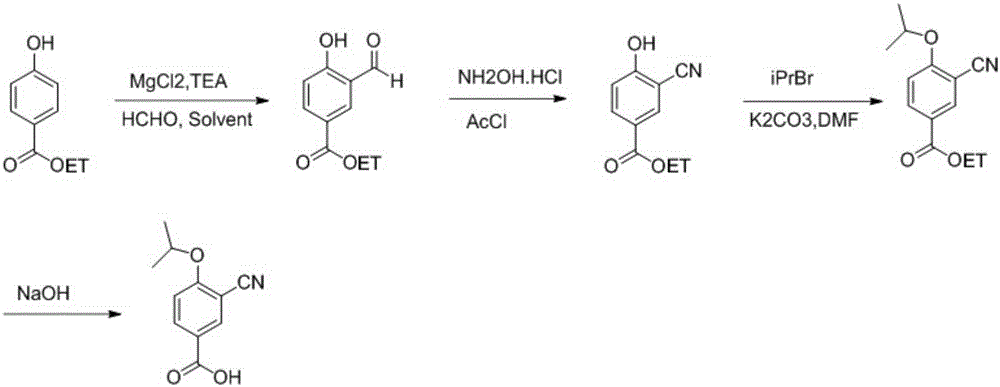
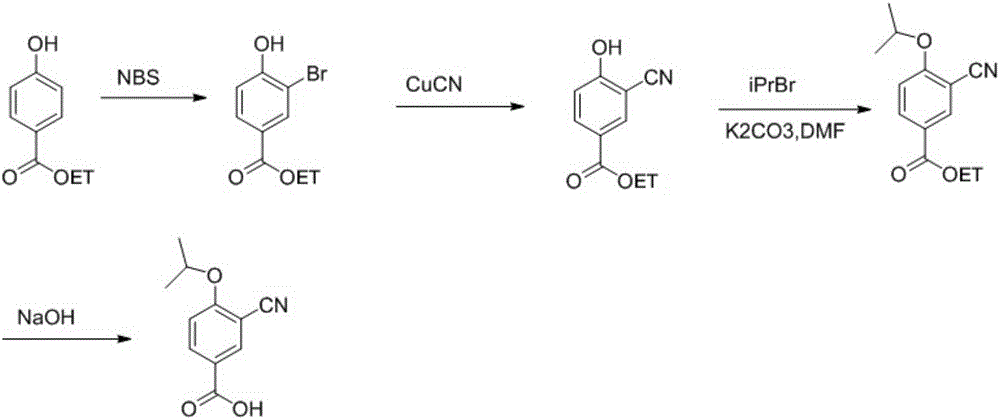
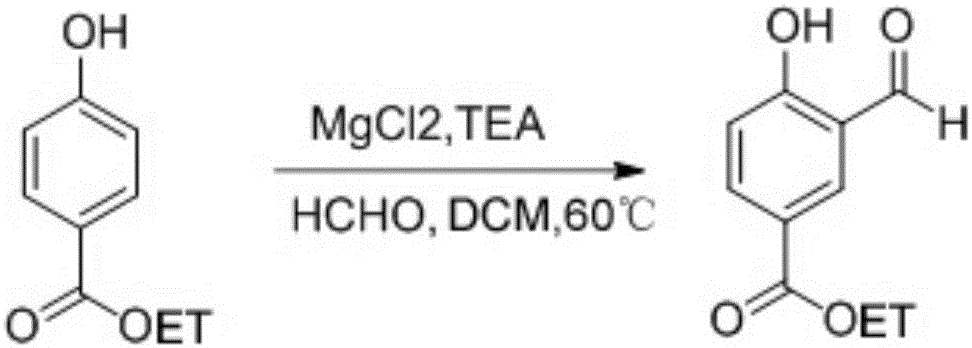
Method for preparing 3-cyano-4-isopropoxybenzoic acid
A technology of isopropoxybenzoic acid and ethyl isopropoxybenzoate, which is applied in the field of preparing 3-cyano-4-isopropoxybenzoic acid, can solve the problems of great harm, and is suitable for mass production , The cost advantage is obvious, and the raw materials are cheap and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0019] 1) Add ethyl p-hydroxybenzoate (2.75kg, 16.4mol), magnesium chloride (3.2kg, 32.8mol), triethylamine (9.3L, 82mol), paraformaldehyde (3.9kg, 131.2mol) into the 50L reaction pot ), dichloromethane (18L), heated in an oil bath at 60°C (inner temperature 44°C) overnight. After cooling to room temperature, slowly add 5 L of concentrated hydrochloric acid diluted aqueous solution, filter off the insoluble matter, extract with DCM 4 times, dry over sodium sulfate, filter and spin dry. directly to the next step.
[0020] 2) Add the raw materials of the previous step into the 50L reaction pot, hydroxylamine hydrochloride (1.14kg) and acetonitrile / N,N-dimethylformamide (10L / 2.5L), add acetyl chloride (1.17L), heat and stir at 80°C for 2 hours . After cooling to room temperature, add 10L of EA, wash with 5L of water twice, reverse extraction with water once, combine the organic layers, dry over sodium sulfate, filter, spin until half dry, a large amount of solid precipitates, d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com