Pentacyclic triterpenes compound with ACC1 protein regulation effect and use of pentacyclic triterpenes compound
A technology of compound and application, applied in the field of pharmacy, can solve the problems such as the anti-proliferation effect of boswellic acid needs to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
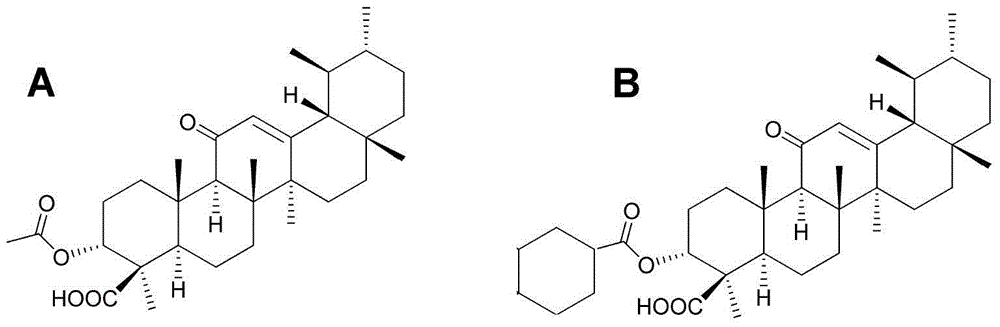
[0091] Embodiment 1: preparation and identification of formula (II) compound (CKBA)
[0092] Add 8 g of acetyl-11-keto-β-boswellic acid (AKBA for short) and 2.63 g of potassium hydroxide (KOH) into a 100 ml two-necked bottle, and add 50 ml of isopropanol as a solvent under nitrogen protection. Heated to reflux for about 6 hours. After the reaction system was cooled to room temperature, the solvent was spin-dried using a rotary evaporator to obtain a white solid. After adding 30 ml of dichloromethane, dilute hydrochloric acid was added to adjust the pH of the mixed system to acidic. Extract the aqueous phase with dichloromethane for 3 times (3*15 ml), collect the dichloromethane solvent, dry with anhydrous magnesium sulfate, spin the solvent to obtain a brown oily product, use petroleum ether:ethyl acetate (90:10) as The eluent was purified by column chromatography to obtain 5.8 g of white solid KBA with a yield of about 78%. Dissolve 1 g of KBA in 10 ml of dichloromethane (co...
Embodiment 2
[0094] Embodiment 2: the preparation of the sodium carboxymethylcellulose suspension of the compound of formula (II)
[0095] (1) Suspension 1
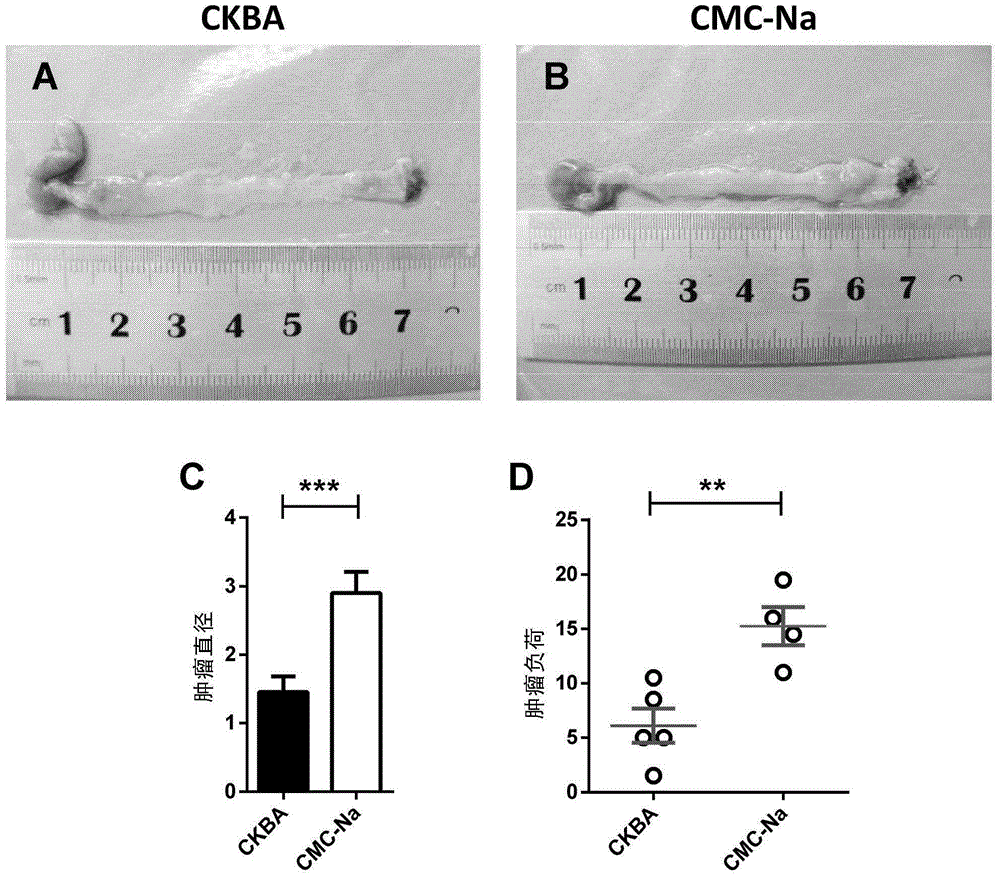
[0096] A certain amount of sodium carboxymethylcellulose (CMC-Na for short) was dissolved in double distilled water overnight to prepare a 0.3% (g / ml) CMC-Na solution. Add a certain amount of CKBA powder to this solution to prepare a 5 mg / ml suspension, and CKBA can be disperse with simple ultrasonic assistance.
[0097] (2) Blank suspension
[0098] A certain amount of sodium carboxymethylcellulose (CMC-Na for short) was dissolved overnight in double distilled water to prepare a 0.3% (g / ml) CMC-Na solution as a blank suspension. The blank suspension was used as a control in animal experiments.
Embodiment 3
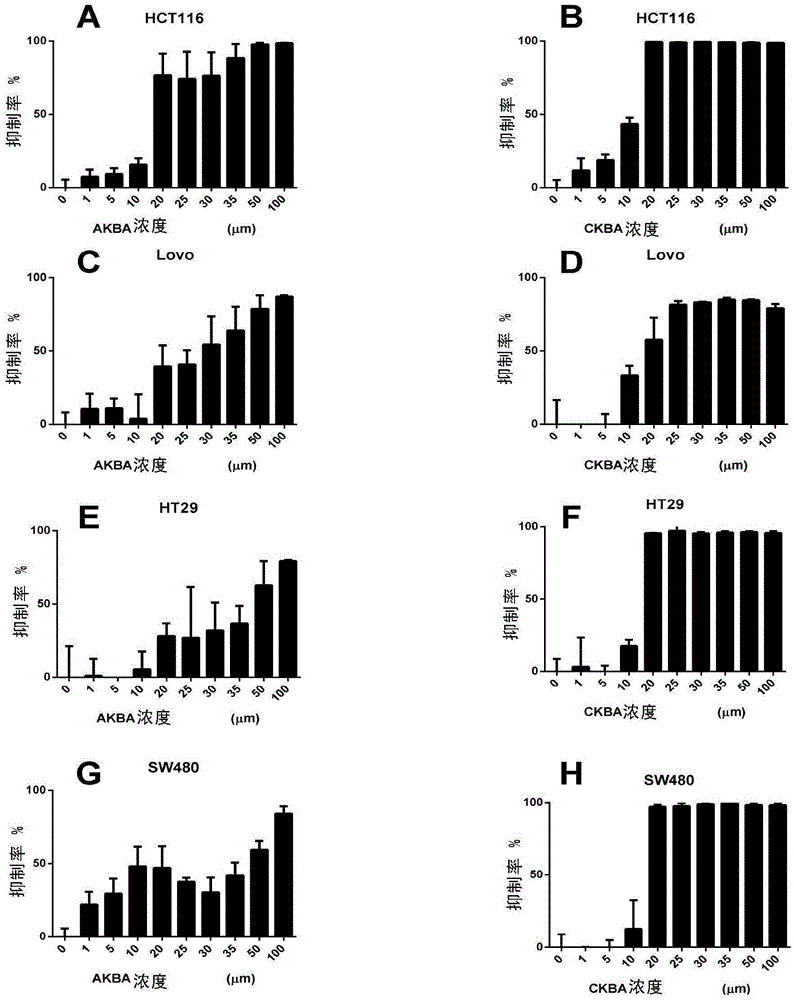
[0099] Embodiment 3: Experiment of inhibiting the growth of human colon cancer cells in vitro
[0100] Take human colon cancer cell lines HCT116, Lovo, HT29 and SW480 (purchased from ATCC) in logarithmic growth phase in 96-well plates, 1.5×10 per well 4 Each cell was added with CKBA and AKBA in gradient concentrations (1, 5, 10, 20, 25, 30, 35, 50, 100 μM) respectively, and the total volume was 100 μl. The control group was added with DMSO at a final concentration of 0.5%. Only 100 microliters of culture medium was added to the blank control wells, and three replicate wells were set up in each group. After 24 hours of culture in the cell incubator, add 10 microliters of CCK-8 solution to each well, and place the culture plate in the incubator (37°C, 5% CO 2 conditions) and incubate in the dark for 4 hours before taking out. The O.D. value at the wavelength of 450nm was measured by a microplate reader. Calculation of inhibition rate: inhibition rate = [1-(O.D. value of exper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com