A kind of low-swellable degradable thermosensitive hydrogel and preparation method thereof
A temperature-sensitive hydrogel and swelling technology, which is used in pharmaceutical formulations, medical formulations with inactive ingredients, and medical formulations containing active ingredients, etc. Dense gel structure, difficult commercial production, etc., to achieve the effects of small hydrodynamic volume and radius of gyration, low viscosity, and low swelling rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
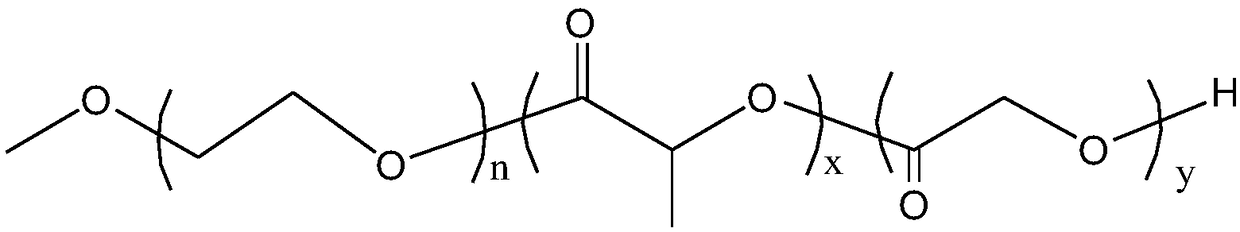
[0070] Weigh 10 g of mPEG (Mn=550) into a vacuum bottle, heat it in an oil bath to 120° C., and remove water under vacuum for 1 h while stirring (vacuum degree is 100 Pa). Then add 58g of D, L-lactide and 5.19g of glycolide, add 0.03% stannous octoate, deoxidize with dry nitrogen, depressurize to a vacuum of 15Pa, seal, and heat up the oil bath to 150°C. Reaction 36h. After the reaction, the crude product was dissolved in a small amount of dichloromethane, then precipitated with excess glacial ether, and the upper solvent was poured off to obtain the copolymer A 1 , vacuum-dried for 24 hours for later use.
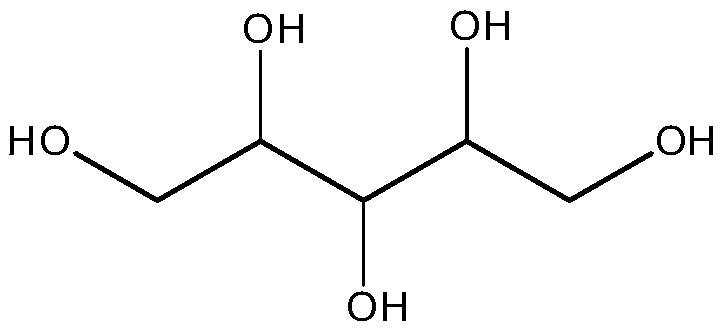
[0071] Dissolve slightly excess 2.59g of glutaric anhydride in 200ml of anhydrous 1,4-dioxane, add 1.49g of 4-lutidine and 2.27g of triethylamine to react at room temperature for 1h, then add 0.552g Xylitol was reacted at room temperature for 32 hours, the product was distilled under reduced pressure to remove 1,4-dioxane, and the distilled product was extracted twice wi...
Embodiment 2
[0075] Weigh 18.18g mPEG (M n =1000) in a vacuum bottle, heated to 120° C. in an oil bath, and removed water under vacuum for 1 h (vacuum degree: 100 Pa) under stirring to remove residual moisture in mPEG. Then add 35g D,L-lactide and 9.40g glycolide, add 0.03% stannous octoate, deoxidize with dry nitrogen, depressurize to a vacuum of 15Pa, seal, and heat the oil bath to 150°C. Reaction 32h. After the reaction, the crude product was dissolved in a small amount of dichloromethane, then precipitated with excess glacial ether, and the upper solvent was poured off to obtain the copolymer A 2 , vacuum-dried for 24 hours for later use.
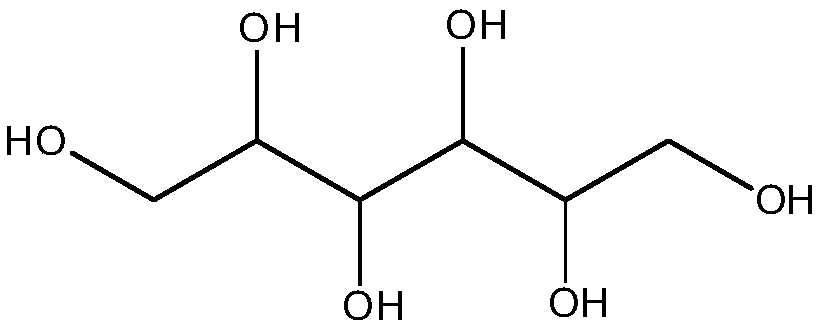
[0076] Dissolve 2.27g of succinic anhydride in 200ml of anhydrous 1,4-dioxane, add 1.50g of 4-lutidine and 2.43g of triethylamine to react at room temperature for 1h, then add 0.552g of xylitol Reacted at room temperature for 24 hours, the product was distilled under reduced pressure to remove 1,4-dioxane, and the distilled product was extracted ...
Embodiment 3
[0080] Weigh 18.18g mPEG (M n=1000) in a vacuum bottle, heated to 120° C. in an oil bath, and removed water under vacuum for 1 h (vacuum degree: 100 Pa) under stirring to remove residual moisture in mPEG. Then add 40g L-lactide and 3.58g glycolide, add 0.03% stannous octoate, deoxidize with dry nitrogen, depressurize to a vacuum of 15Pa, seal, heat the oil bath to 130°C, and react for 48h . After the reaction, the crude product was dissolved in a small amount of dichloromethane, then precipitated with excess glacial ether, and the upper solvent was poured off to obtain the copolymer A 3 , vacuum-dried for 24 hours for later use.
[0081] Dissolve 2.27g of succinic anhydride in 200ml of anhydrous 1,4-dioxane, add 1.50g of 4-lutidine and 2.27g of triethylamine to react at room temperature for 1h, then add 0.552g of xylitol After reacting at room temperature for 36 hours, the product was distilled under reduced pressure to remove 1,4-dioxane, and the distilled product was extr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com