The preparation method of α-phenethyl alcohol
A technology of phenylethyl alcohol and acetophenone, which is applied in the field of preparation of α-phenylethyl alcohol, can solve the problems of high cost, low synthesis selectivity, easy environmental pollution, etc., achieve short reaction time, mild reaction conditions, and reduce industrial production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] After adding 0.8g of 16.7% Co / mordenite catalyst, 5ml of acetophenone, and 160ml of solvent water into a 250ml stainless steel reactor, seal it, replace it with nitrogen and hydrogen for three times, feed hydrogen, and control the reaction pressure to 2MPa. It is 800r / min, and the reaction temperature is 100°C. After 6 hours of reaction, the product at the outlet of the reactor is separated from the liquid product through a gas-liquid separator, and the liquid product is analyzed by a gas chromatography spectrometer. , the selectivity of α-phenylethanol is 100%.
[0026] Except having changed the reaction conditions as shown in Table 1, it reacted and measured in the same manner as Example 1. Reaction condition and result are as shown in embodiment 2-4 in table 1:
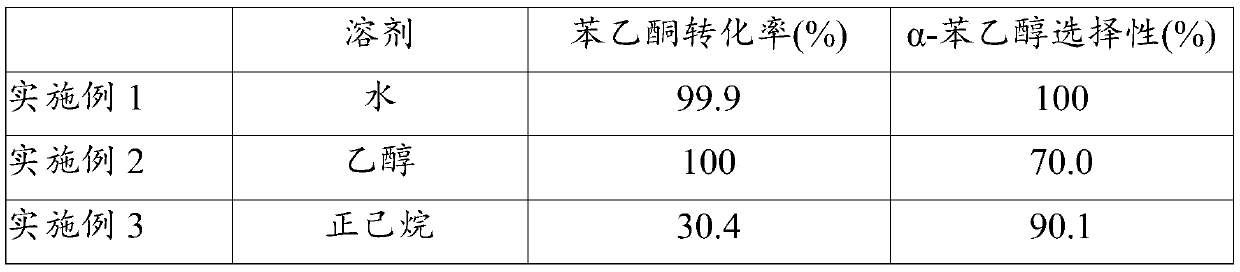
[0027] Table 1 Hydrogenation of acetophenone on Co / mordenite catalysts to produce phenylethyl alcohol in different solvents
[0028]
[0029]
[0030] As can be seen from Table 1, when water is used ...
Embodiment 8 to Embodiment 11
[0036] Embodiment 8 to embodiment 11 investigate the impact of reaction time on conversion rate and selectivity
[0037] Except having changed the reaction conditions as shown in Table 3, it reacted and measured in the same manner as Example 1. Reaction condition and result are as shown in embodiment 8-11 in table 3:
[0038] Table 3 The influence of different reaction times in solvent water on the reaction of acetophenone hydrogenation to phenylethanol
[0039] time / h Acetophenone conversion rate (%) α-Phenethyl alcohol selectivity (%) Example 1 6 99.9 100 Example 8 2 52.3 100 Example 9 4 85.0 100 Example 10 8 100 99.8 Example 11 10 100 99.3
[0040] It can be seen from Table 3 that as the reaction time prolongs, the conversion rate of acetophenone increases, and the reaction time is preferably 6 to 10 hours.
[0041] Embodiment 12 to embodiment 16 investigates the influence of reaction temperature on conversion ra...
Embodiment 17
[0048] After adding 0.8g of 16.7% Co / Hβ catalyst, 5ml of acetophenone, and 160ml of solvent water in a 250ml stainless steel reactor, seal it, replace it with nitrogen and hydrogen for three times, feed hydrogen, control the reaction pressure to 2MPa, and the rotating speed is 800r / min, reaction temperature 100 DEG C, after reacting for 6 hours, the product of reactor outlet is separated liquid product through gas-liquid separator, and liquid product is analyzed with mass spectrometry instrument, and the transformation rate of acetophenone is 97.1%, The selectivity of α-phenylethanol is 97.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com