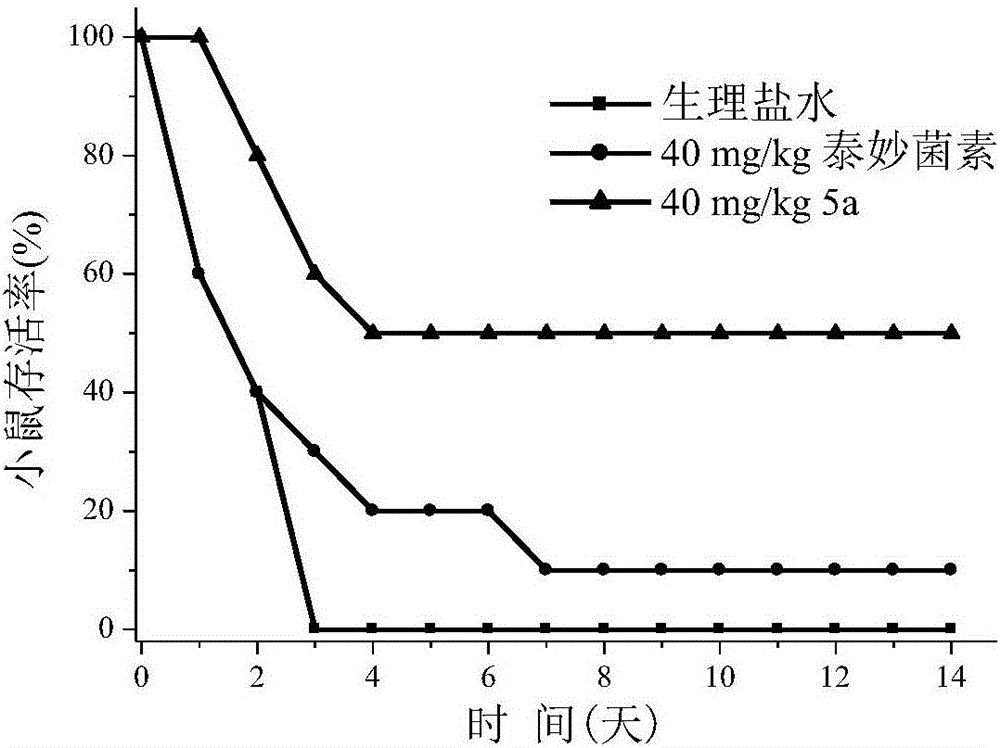
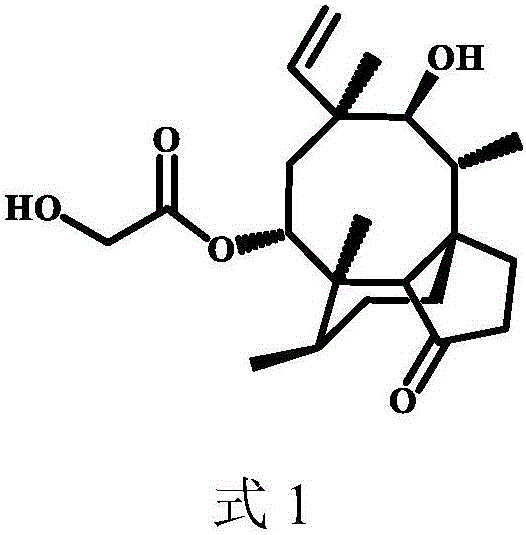
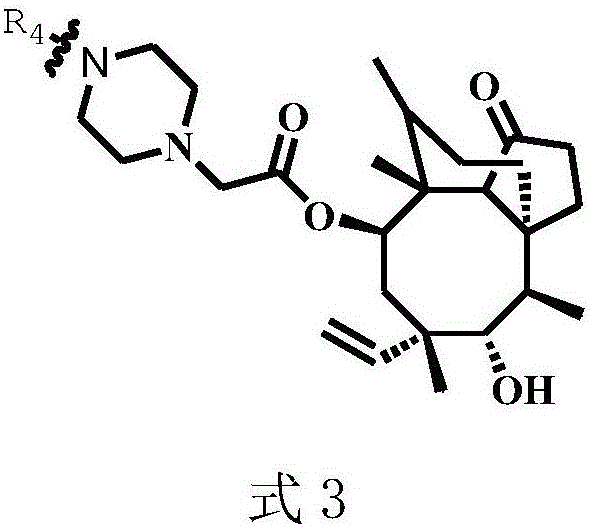
Pleuromulin derivative with acyl piperazine side chain and preparing method and application of pleuromulin derivative
A technology of pleuromutilin and piperazine group, which is applied in the field of pleuromutilin derivatives and their preparation, and can solve the problems of rare bacteria resistant to pleuromutilin antibacterial drugs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1: Synthesis of 14-O-1-(4-(2-N-methylpiperazinylacetyl)) piperazinyl muulin (compound 1a)
[0084] 1g (1.91mmol) of intermediate III was dissolved in 10mL of acetonitrile, 0.53g (3.82mmol) of potassium carbonate and 0.233mL (2.1mmol) of 1-methylpiperazine were added, and heated and stirred at 70°C for 3h to obtain the target product. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above crude product-silica gel powder mixture was purified by column chromatography (200-300 The mesh silica gel powder is the stationary phase, and ethyl acetate:methanol=20:1 is the mobile phase), to obtain the product 14-O-1-(4-(2-N-methylpiperazinylacetyl))piperazinyl The pure product of Lin (compound 1a). Yield 40.78%. HR-MS(ESI): Calcd.for C 33 h 55 N 4 o 5 (M+H + ):587.4167; Found: 665.41803.
Embodiment 2
[0085] Example 2: Synthesis of 14-O-1-(4-(2-methylphenylpiperazinylacetyl)) piperazinyl muulin (compound 2a)
[0086] 1g (1.91mmol) of intermediate III was dissolved in 10mL of acetonitrile, 0.53g (3.82mmol) of potassium carbonate and 0.37g (2.1mmol) of 2-methylphenylpiperazine were added, and heated and stirred at 70°C for 3h to obtain the target product. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above crude product-silica gel powder mixture was purified by column chromatography (200-300 Mesh silica gel powder is stationary phase, and ethyl acetate:methanol=20:1 is mobile phase), obtains product 14-O-1-(4-(2-methylphenylpiperazinylacetyl))piperazinyl The pure product of Lin (compound 2a). Yield 46.74%. HR-MS(ESI): Calcd.for C 39 h 59 N 4 o 5 (M+H + ): 663.4480; Found: 663.4485.
Embodiment 3
[0087] Example 3: Synthesis of 14-O-1-(4-(3-methylphenylpiperazinylacetyl)) piperazinyl muulin (compound 3a)
[0088] 1g (1.91mmol) of intermediate III was dissolved in 10mL of acetonitrile, 0.53g (3.82mmol) of potassium carbonate and 0.36mL (2.1mmol) of 3-methylphenylpiperazine were added, and heated and stirred at 70°C for 3h to obtain the target product. The obtained mixed solution was rotary evaporated to dryness, and the mixture was redissolved in dichloromethane, and 1 g of 100-200 mesh silica gel was added to mix thoroughly. After the solvent evaporated, the above crude product-silica gel powder mixture was purified by column chromatography (200-300 Mesh silica gel powder is stationary phase, and ethyl acetate:methanol=20:1 is mobile phase), obtains product 14-O-1-(4-(3-methylphenylpiperazinylacetyl))piperazinyl The pure product of Lin (compound 3a). Yield 60.56%. HR-MS(ESI): Calcd.for C 39 h 59 N 4 o 5 (M+H + ): 663.4480; Found: 663.4470.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com