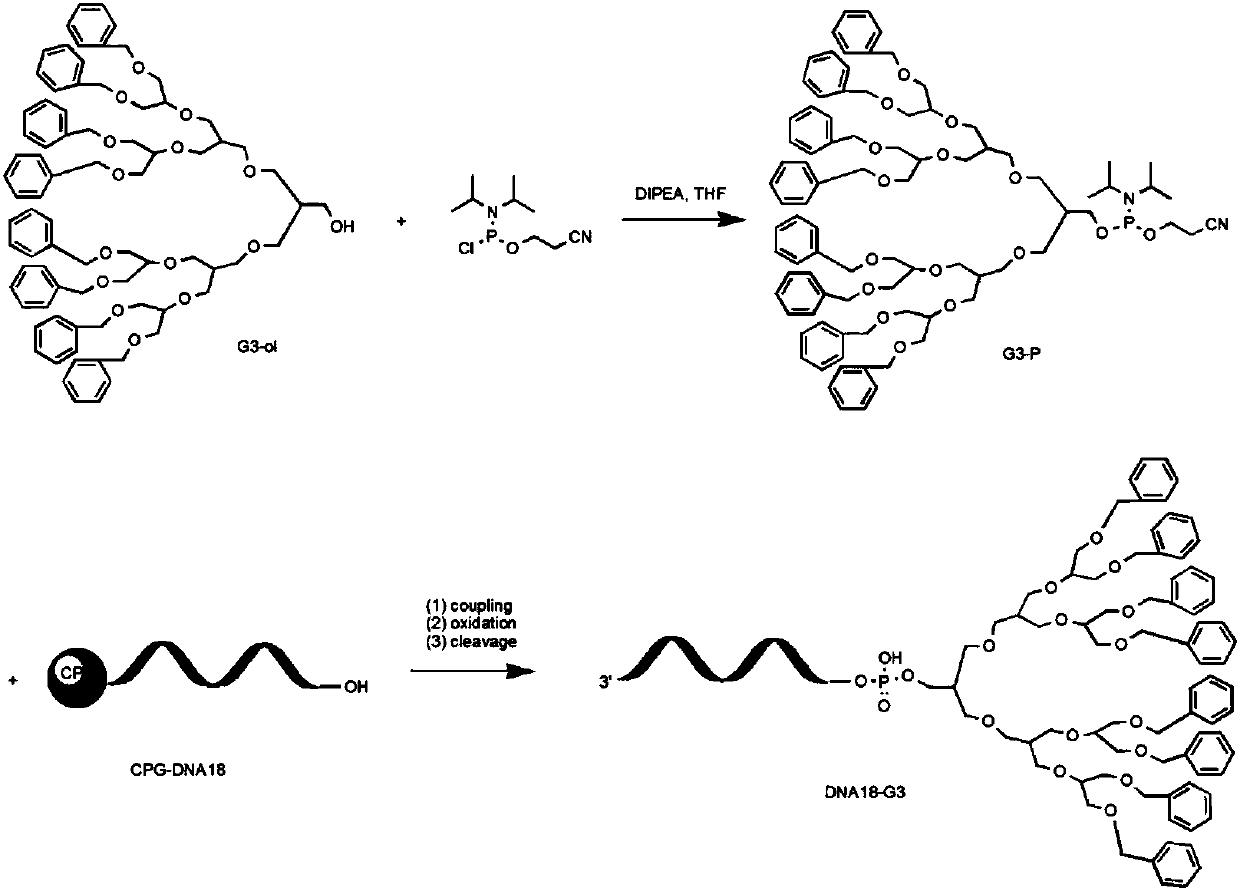
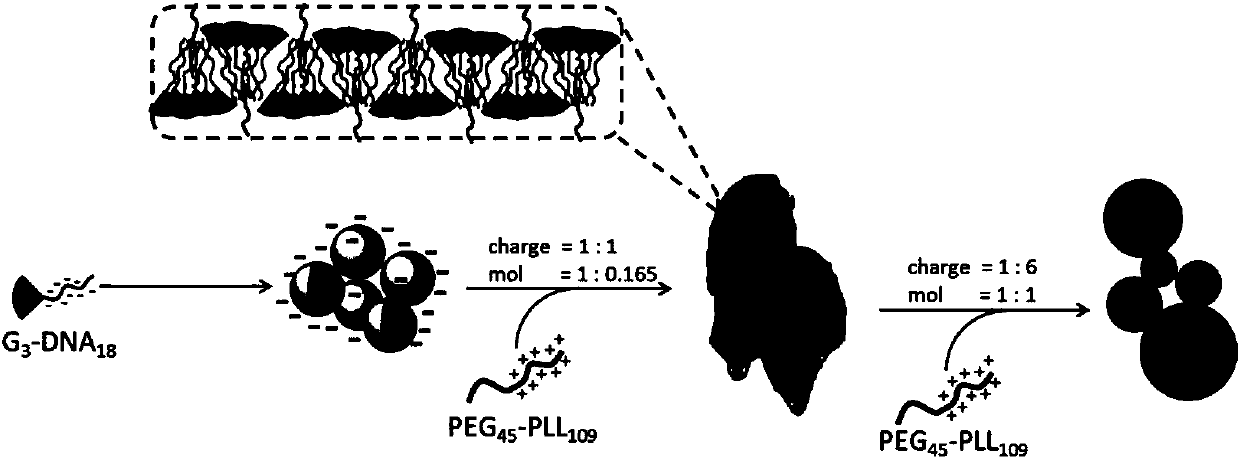
Supramolecular assembly and preparation method and morphological control method thereof
A technology of supramolecular assembly and morphology, applied in the field of supramolecular, to avoid the effect of shielding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of α-lysine-N-carboxy-anhydride (Lys(Z)-NCA)
[0038] (1) L-lysine (H-Lys(Z)-OH) and 20% excess triphosgene were reacted in tetrahydrofuran (THF) solvent at 50°C until clarified and then reacted for one hour to convert into L - Structure of lysine-N-carboxy-cyclic anhydride (Lys(Z)-NCA).
[0039] (2) The obtained L-lysine-N-carboxy-cyclic acid anhydride (Lys(Z)-NCA) monomer crude product was recrystallized and purified three times from THF and n-hexane in a glove box to obtain the final product for use.
Embodiment 2
[0040] Embodiment 2: synthetic polyethylene glycol-b-poly-L-lysine (PEG-b-PLL)
[0041] (1) First monomethoxypolyethylene glycol primary amine (mPEG-NH 2 ,M n =2000g mol -1 ) Vacuum-dried in a Schlenk bottle at 50°C in an oil bath for about 6 hours, and then cooled to room temperature;
[0042] (2) The L-lysine-N-carboxyl-cyclic anhydride (Lys(Z)-NCA) of Example 1 was dissolved in dry dimethylformamide (DMF) in a glove box, and then extracted with a syringe Then transfer to the Schlenk bottle and react under nitrogen protection and room temperature; after using FT-IR (Fourier Transform Infrared Spectrometer) spectrum to confirm that the reaction is complete, get a small amount of sample and prepare 5mg / mL for SEC / LLS (Size Exclusion Chromatography / laser scattering) test to determine polydispersity (PDI) and molecular weight (M n );
[0043] (3) After excessive ether precipitation, dissolve the product with a small amount of trifluoroacetic acid (TFA), add 5 times the equ...
Embodiment 3
[0045] Embodiment 3: prepare polyethylene glycol-b-poly-L-lysine (PEG 45 -b-PLL 109 ) solution
[0046] The PEG of embodiment 2 45 -b-PLL 109 Dissolved in water, configured into a solution with a molar concentration of 9.175 μM, and the measured molar concentration of the positive charge of the protonated amino group of the polylysine side chain in the solution is 1 mM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com